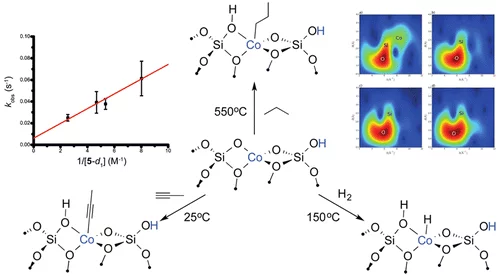
The activation and conversion of hydrocarbons is one of the most important challenges in chemistry. Transition-metal ions (V, Cr, Fe, Co, etc.) isolated on silica surfaces are known to catalyze such processes. The mechanisms of these processes are currently unknown but are thought to involve C–H activation as the rate-determining step. Here, we synthesize well-defined Co(II) ions on a silica surface using a metal siloxide precursor followed by thermal treatment under vacuum at 500 °C. We show that these isolated Co(II) sites are catalysts for a number of hydrocarbon conversion reactions, such as the dehydrogenation of propane, the hydrogenation of propene, and the trimerization of terminal alkynes. We then investigate the mechanisms of these processes using kinetics, kinetic isotope effects, isotopic labeling experiments, parahydrogen induced polarization (PHIP) NMR, and comparison with a molecular analog. The data are consistent with all of these reactions occurring by a common mechanism, involving heterolytic C–H or H–H activation via a 1,2 addition across a Co–O bond.
Contact
Dr Olga SafonovaSuperXAS beamline
Laboratory for Synchrotron Radiation and Femtochemistry (LSF)
Swiss Light Source, Paul Scherrer Intitute
5232 Villigen-PSI, Switzerland Telephone: +41 56 310 58 05
E-mail: olga.safonova@psi.ch
Original Publication
C–H Activation on Co,O Sites: Isolated Surface Sites versus Molecular AnalogsDeven P. Estes, Georges Siddiqi, Florian Allouche, Kirill V. Kovtunov, Olga V. Safonova, Alexander L. Trigub, Igor V. Koptyug, and Christophe Copéret
JACS, 21 October 2016
DOI: 10.1021/jacs.6b08705