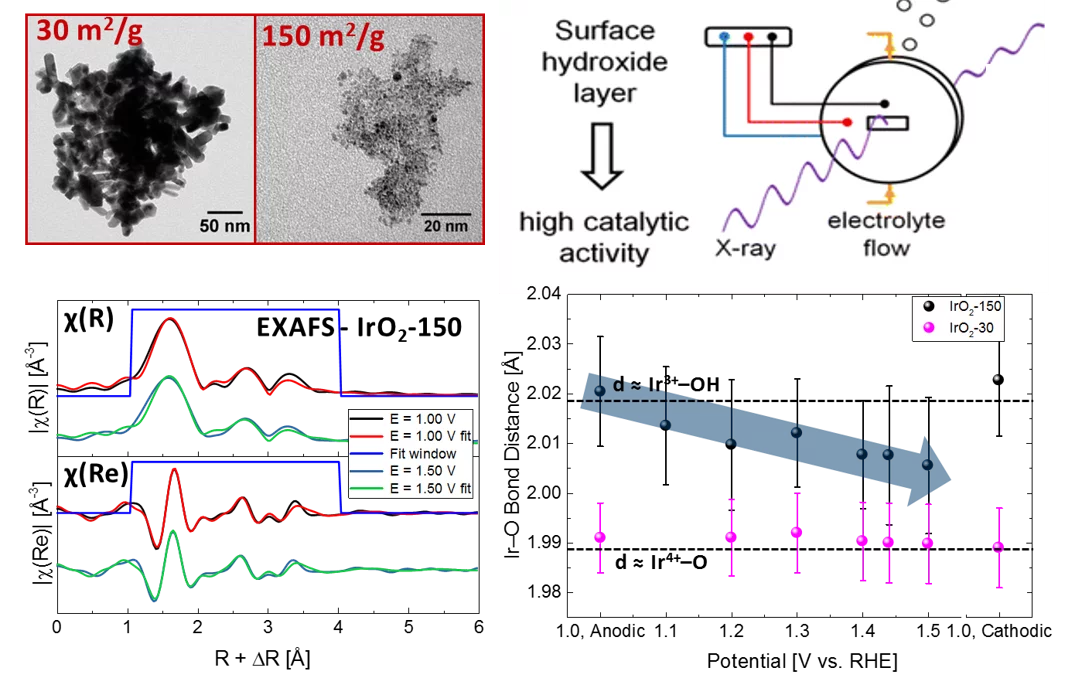
Iridium oxides with different surface area (30 and 150 m2/g) have been investigated by operando X-ray absorption spectroscopy (XAS). While the operando XAS analysis provided little information regarding the nature of IrO2-30 due to the larger size of the particles, valuable insight was gained regarding the surface of IrO2-150. IrO2-150 initially exists in a mixed Ir3+/Ir4+ oxidation state, but transitions to a higher oxidation state upon anodic polarization above 1.00 V vs. RHE take place. These transitions were followed by a shift of the edge energy in the XANES spectra and also by monitoring the Ir−O distances in the first coordination sphere obtained from the fitted EXAFS spectra recorded as a function of the applied potential. These hydroxo species are likely converted to oxide species at the typical potential of the oxygen evolution reaction (OER, i.e., 1.4-1.5 V vs RHE). Overall, this study shows that small IrO2 nanoparticles with a hydroxide surface layer are particularly efficient OER catalysts.
Please refer to Chem. Mater. 2016, 28, 6591−6604 for further information.