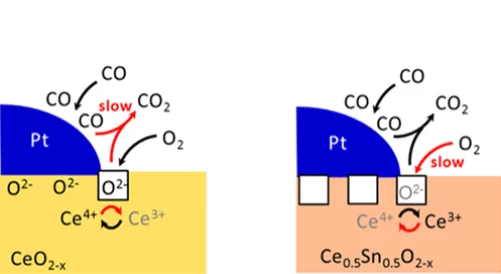
The metal–oxide interface is the place where many catalytic reactions take place. However, it is challenging to detect in situ active sites involved in the catalytic mechanism, due to their low amount and/or short lifetime. In this work, the impact of cerium oxide reducibility on the mechanism of low-temperature CO oxidation over platinum nanoparticles is investigated combining different spectroscopic techniques. The reducibility of Ce4+ increases at the Pt/Ce0.5Sn0.5O2 interface, compared to that of Pt/CeO2. As a consequence, the catalytic CO oxidation rate increases, the apparent activation energy decreases and the reaction order in oxygen increases. Operando time-resolved X-ray absorption spectroscopy suggests that the Ce4+ reduction rate at the Pt/Ce0.5Sn0.5O2 interface is accelerated, while the Ce3+ oxidation rate becomes rate-limiting. Importantly, no reduction of Sn4+ in the ceria–tin solid solution and no formation of Pt/Sn alloys were detected under relevant reaction conditions using in situ X-ray absorption spectroscopy, ambient-pressure X-ray photoelectron spectroscopy, and infrared spectroscopy. This work provides a better understanding on the reactivity of interfaces. It demonstrates that the reducibility of the oxide close to a metal strongly influences catalytic rates, which provides ideas for the design of better catalysts.