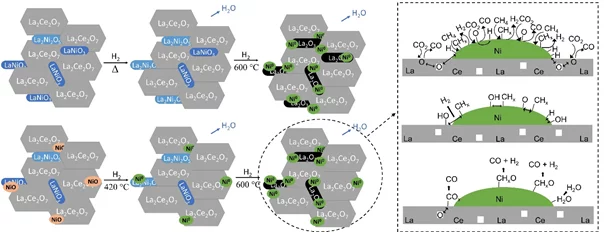
Dry reforming of methane is a promising process to generate syngas with a low H2/CO ratio. So far, the main issues observed on catalytic materials during the reaction were the formation of coke and metal active sites sintering. Pyrochlores and perovskites are stable and promising materials displaying properties that can overcome these challenges due to the presence of well dispersed active metal and oxygen vacancies. La2Ce2O7 and LaNiO3 mixed oxides containing 5 wt% of nickel were synthesized by two different methods. Their activation in hydrogen was followed by in situ X-ray diffraction, ambient pressure X-ray photoelectron spectroscopy, and X-ray absorption near edge structure. An oxygen-deficient perovskite La2Ni2O5 was identified already at room temperature. Both catalysts were stable during the performance test under low-(600°C) and high-(850°C) temperature DRM reaction, thanks to a negligible carbon accumulation on the catalyst surface. The high amount of electrophilic oxygen species, oxygen vacancies and basic sites detected correlates with the high catalytic activity. The material synthesized by hydrothermal method showed the highest conversion and yield. The better performance in this catalyst was related to high amount of intermediate basic sites, oxygen vacancies and greater interaction between nickel and cerium. The reaction mechanism proposed in these materials takes into account the intermediates CHx and adsorbed O/OH, which were observed by means of photoelectron spectroscopy.