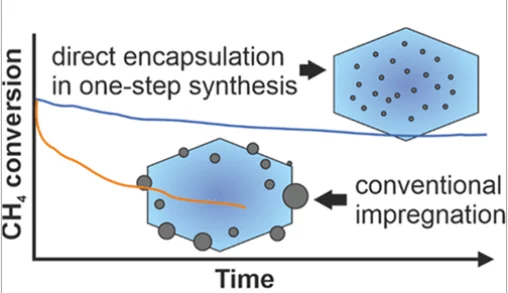
The stability of catalytically active species is one of the most critical factors determining the reaction performance. Zeolites are suitable supports for dispersed active metal nanoparticles. Palladium supported on zeolite catalysts show high activity towards methane oxidation but deactivate rapidly under the reaction conditions due to palladium sintering. Several approaches have been attempted, in order to stabilize palladium. In this work, using an amine-based ligand to stabilize palladium during the zeolite synthesis, highly dispersed palladium oxide clusters (1.8–2.8 nm) encapsulate in the microporous channels and voids of the nanosized silicalite-1 crystals. The synthesis conditions influence the encapsulation degree and the size distribution of metal particles. Thanks to the encapsulation effect of small palladium oxide clusters, together with the inherent properties of silicalite-1 such as low acidity, high hydrophobicity, and high hydrothermal stability, the optimized Pd@silicalite-1 catalyst outperforms the traditional Pd-based catalysts prepared by wetness impregnation, exhibiting both high activity and better stability in the lean methane oxidation reaction.