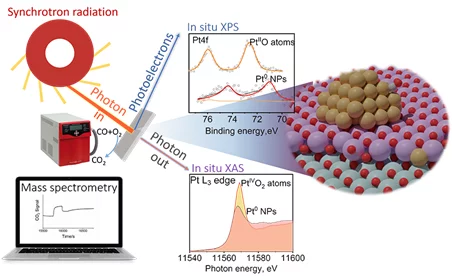
Catalytic systems based on supported noble metals are extensively studied because of their widespread application. Discussions remain about the nature of the active species, whether they are atomically dispersed or nanoparticles, and their reactivity. In this work, combining in situ/operando spectroscopies with theoretical modeling, we propose a phase diagram to follow the evolution of atomically dispersed platinum on ceria. Such species reversibly change from PtIVO2 to PtIIO as a function of temperature and oxygen partial pressure. The phase diagram helps identify the stability domain of each species, while spectroscopies provide a quantitative evaluation depending on the reaction conditions. Finally, our results show that high-temperature activation in the presence of steam of supported atomically dispersed platinum enhances the activity toward low-temperature carbon monoxide oxidation because it promotes aggregation into nanoparticles. This work highlights the structure–activity relationship in supported metal catalysts and proposes a suitable approach to determine the amount of each species before the investigation of the reaction mechanism.