Metal-Organic Frameworks - Synthesis towards unique catalytic performance
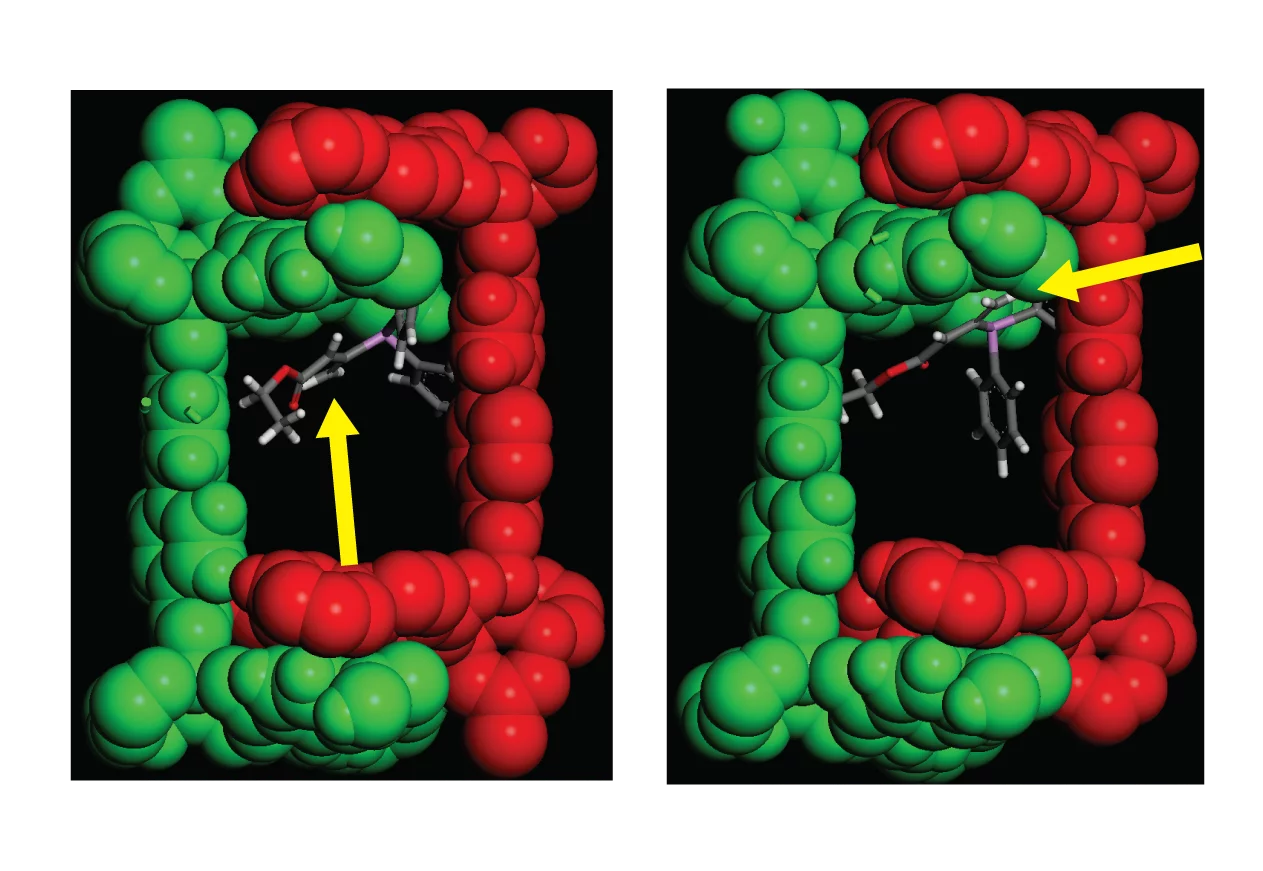
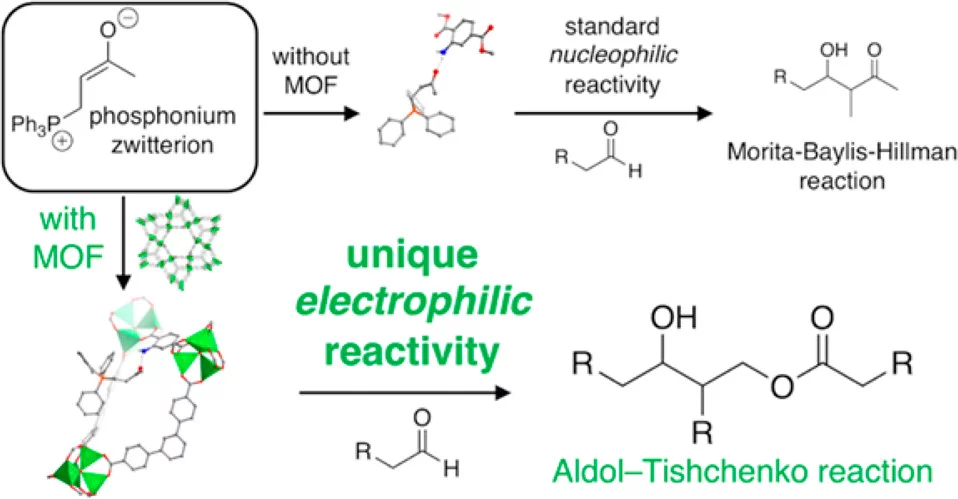
Metal-organic frameworks (MOFs), materials obtained by the combination of organic building blocks with metal or metal clusters, are one of the fastest growing classes of materials in the last decades. They are uniquely versatile from a chemical point of view as organic linkers are integral to their structure. This feature allows the tuning of the pore size and of the sorption properties, as well as functionalization through isoreticular and post-synthetic techniques. The aim of MOF research of the Syncat is to exploit the unique features of MOFs to drive reactions that are impossible to achieve with existing homogeneous or heterogeneous catalysts. The long-term goal is to make MOFs a viable tool for organic synthesis and for the activation of small molecules. The main idea is to exploit the confinement of active sites and molecules within porous cages in a MOF to get catalysts with unique properties, inspired in model by enzymatic catalysis and to achieve selectivity not obtainable otherwise. Among the many MOF catalytic applications we work actively on:
- Hydroformylation of olefins
- Cross coupling reactions
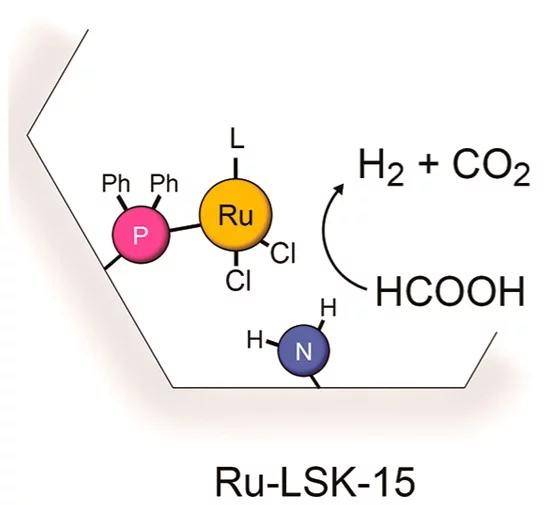
- CO2 Hydrogenation
Selected Publications on The Topic
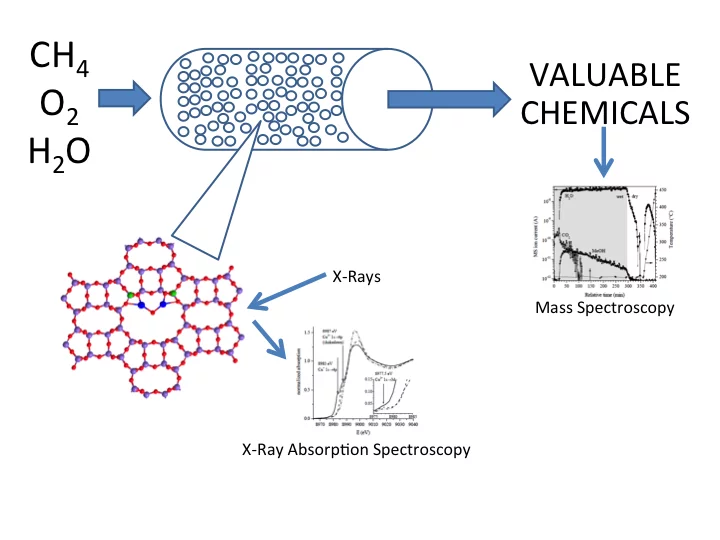
Catalytic conversion of methane to methanol with Cu-zeolite catalysts
Methane is often found in crude oil reservoirs, escapes during oil production and is flared producing CO2. The production of methanol from methane, right at the source, could transform the volatile gas into an easier-to-handle fuel. Storage and trasport to the consumer as methanol would be both technically and economically viable. The direct oxidative conversion of methane to methanol is considered one of the "Holy Grail" of chemistry and is difficult, because of the high reactivity of the formed methanol compared to the starting reactant. One way to circumvent this problem is to form a stable complex of the initially activated methane. In this way, the oxidation product is stabilized and prevented from further reaction to CO2. Activated Cu zeolites are known to activate methane to an intermediate product that can be desorbed as methanol.
Selected publications on the topic
| Tomkins, P., Mansouri, A., Sushkevich, V. L., van der Wal, L. I., Bozbag, S. E., Krumeich, F., Ranocchiari, M., & van Bokhoven, J. A. (2019). Increasing the activity of copper exchanged mordenite in the direct isothermal conversion of methane to methanol by Pt and Pd doping. Chemical Science, 10(1), 167-171. http://dx.doi.org/10.1039/c8sc02795a |  |
| Sushkevich, V. L., Palagin, D., Ranocchiari, M., & van Bokhoven, J. A. (2017). Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science, 356(6337), 523-+. http://dx.doi.org/10.1126/science.aam9035 |  |
| Ravi, M., Ranocchiari, M., & van Bokhoven, J. A. (2017). The Direct Catalytic Oxidation of Methane to Methanol-A Critical Assessment. Angew Chem Int Edit, 56(52), 16464-16483. http://dx.doi.org/10.1002/anie.201702550 |  |
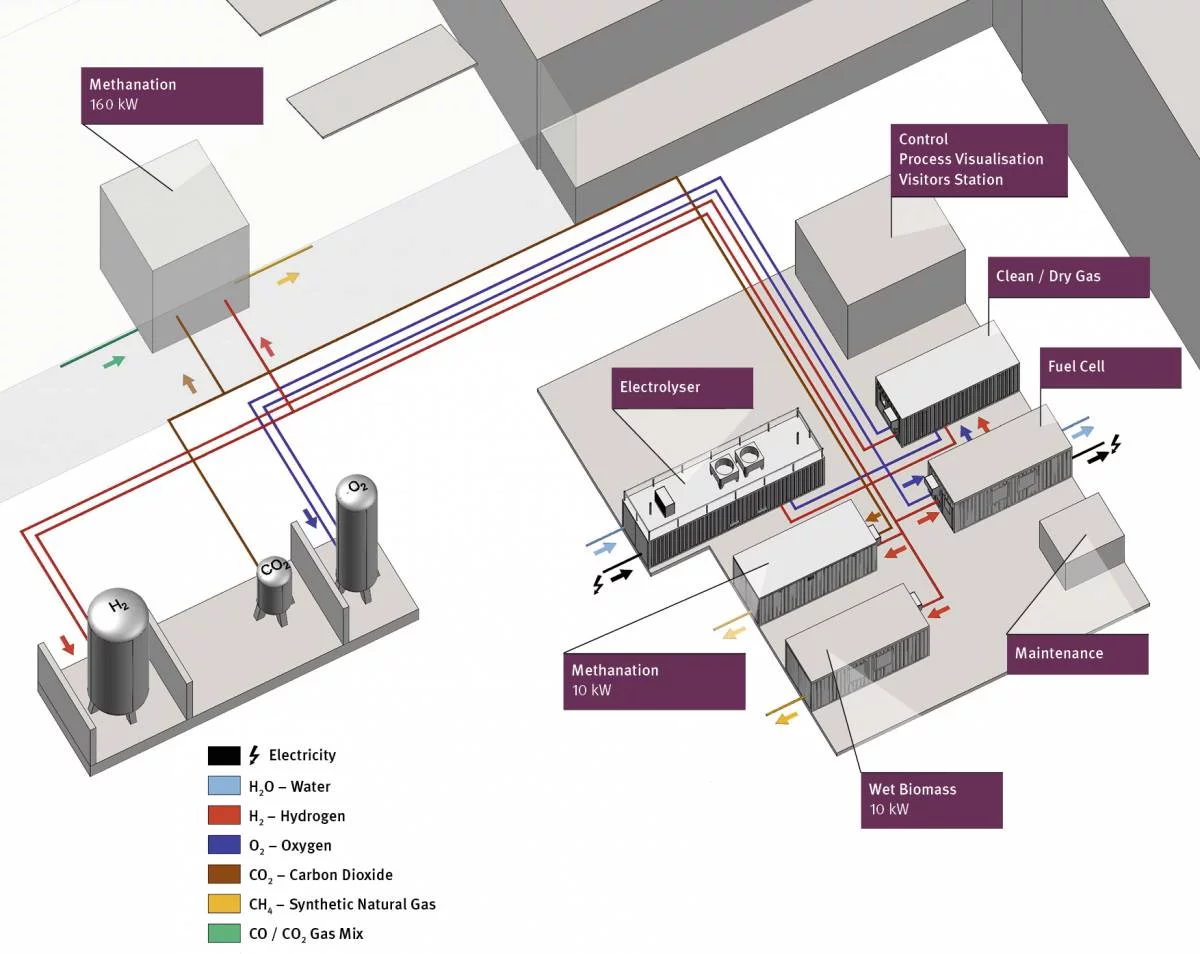
The ESI platform is a unique opportunity to bring fundamental research to the applied field. As part of the Catalysis Team, we collaborate with several platforms within ESI to synthesize the catalytic materials of the future. We carry out fundamental science with overlap with:
- the methanation platform
- the wet biomass platform
- the electrochemistry lab
Synfuel
Within Synfuel project we want to bring catalyst design and development for the sustainable production of aviation fuel. We carry out fundamental science aimed at discovering catalytic materials that will allow to fly in a carboneutral way.