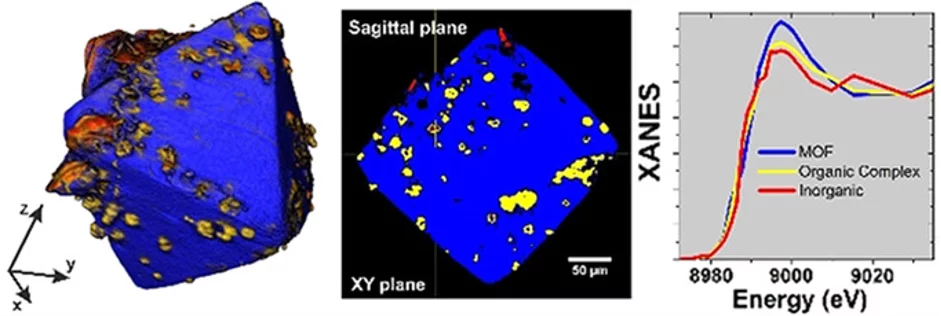
Full-field X-ray absorption tomography reveals the chemical structure of defects in metal-organic frameworks
A better understanding of the relationship between defect-engineered MOF synthesis conditions and the structure of the resulting material is essential. Through the use of full-field X-ray absorption near-edge structure microscopy and tomography, we were able to visualize the chemical heterogeneity in defect-engineered HKUST-1 MOF single crystals. Tomographic analysis revealed a spatially non-uniform incorporation of the defective linker, leading to cluster formation of a secondary coordination polymer throughout the crystals. These clusters differ from the surrounding HKUST-1 framework in metal concentration and copper coordination. Although the frequency of clusters is positively correlated with the defective linker concentration in the crystallisation environment, clusters can equally be found in HKUST-1 crystals synthesized in absence of a defective linker. This suggests that secondary coordination polymer formation is associated with a post-synthesis collapse of the MOF lattice driven by an increased density of crystallographic defects and in turn enhanced by the zonation of defective linkers.
The visualisation of heterogeneities, including the detection of secondary phases of reduced copper coordination, within entire defect-engineered MOF crystals, allowed us to provide an unprecedented point of view towards explaining the structure-property relationship of defect-engineered MOFs and introduce full-field X-ray absorption near-edge structure microscopy and tomography as a powerful tool to investigate the chemistry of such important materials and characterize MOFs in the 3D space.
The micropores of metal-organic frameworks increase selectivity in homogeneous catalysis by adsorption-driven kinetic modulation
Heterogeneous catalysts are often preferred in industrial settings because of their robustness and lower operating costs, but homogenous catalysts still dominate when high selectivity is needed—finding superior heterogeneous catalysts has been a challenge. A collaboration between the Paul Scherrer Institute's experimental Syncat Group, led by Marco Ranocchiari, and EPFL’s Laboratory of Molecular Simulation, a computational group led by Berend Smit, has shown how micropores in metal-organic frameworks (MOFs) can enhance selectivity to levels that cannot be achieved with existing catalysts. Though the findings have significant potential in the production of aldehydes, the easy experimental protocol and the chemical and structural flexibility of MOFs means that the approach represents a powerful tool for designing selective catalytic heterogeneous processes in the fine chemical industry overall.
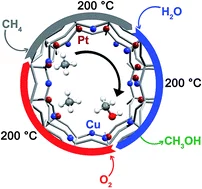
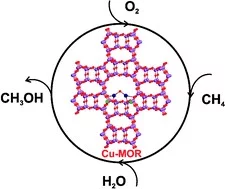
Increasing the activity of copper exchanged mordenite in the direct isothermal conversion of methane to methanol by Pt and Pd doping
PtCu- and PdCu–mordenite allow for isothermal reaction at 200 °C for the stepwise methane to methanol conversion with comparably high yields. In contrast to traditional Cu-zeolites, these materials are more reactive under isothermal conditions than after high temperature activation.
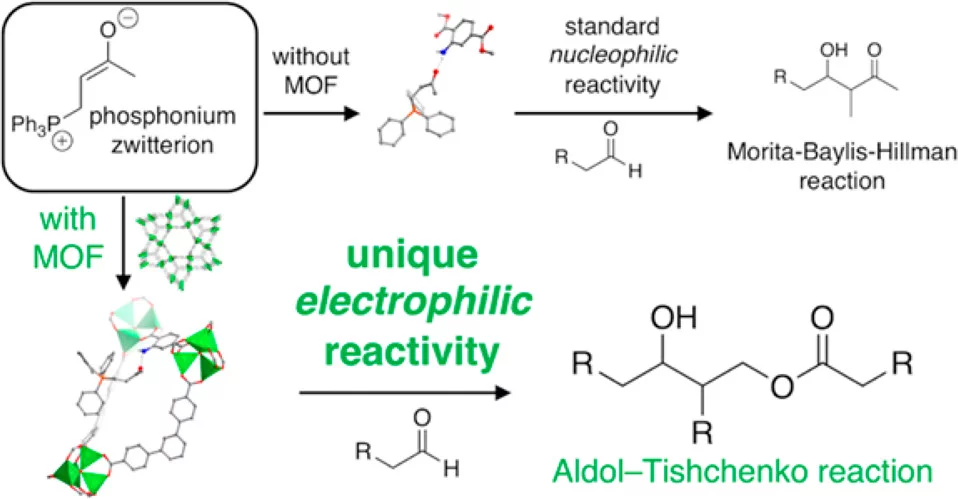
Metal–Organic Frameworks Invert Molecular Reactivity: Lewis Acidic Phosphonium Zwitterions Catalyze the Aldol-Tishchenko Reaction
The influence of metal–organic frameworks (MOFs) as additives is herein described for the reaction of n-alkyl aldehydes in the presence of methylvinylketone and triphenylphosphine. In the absence of a MOF, the expected Morita–Baylis–Hillman product, a β-hydroxy enone, is observed. In the presence of MOFs with UMCM-1 and MOF-5 topologies, the reaction is selective to Aldol-Tishchenko products, the 1 and 3 n-alkylesters of 2-alkyl-1,3-diols, which is unprecedented in organocatalysis. The (3-oxo-2-butenyl)triphenylphosphonium zwitterion, a commonly known nucleophile, is identified as the catalytic active species. This zwitterion favors nucleophilic character in solution, whereas once confined within the framework, it becomes an electrophile yielding Aldol-Tishchenko selectivity. Computational investigations reveal a structural change in the phosphonium moiety induced by the steric confinement of the framework that makes it accessible and an electrophile.
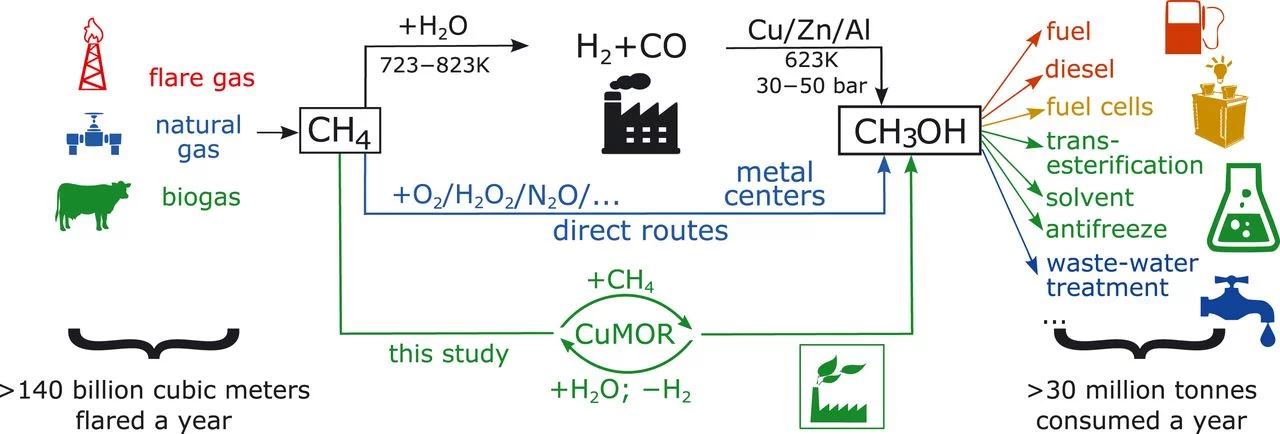
Selective anaerobic oxidation of methane enables direct synthesis of methanol
Methanol production is an expensive, energy-intensive process that initially overoxidizes methane to carbon monoxide. Sushkevich et al. used copper sites in a zeolite to oxidize methane to methoxy intermediates; they then added water to release methanol and hydrogen while reoxidizing the copper. This inexpensive process could prove useful at gas well sites for producing an easily stored and transported liquid from excess gas that at present is burned away.
The Direct Catalytic Oxidation of Methane to Methanol—A Critical Assessment
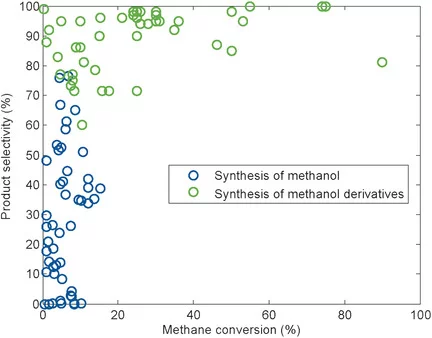
Despite the large number of disparate approaches for the direct selective partial oxidation of methane, none of them has translated into an industrial process. The oxidation of methane to methanol is a difficult, but intriguing and rewarding, task as it has the potential to eliminate the prevalent natural gas flaring by providing novel routes to its valorization. This Review considers the synthesis of methanol and methanol derivatives from methane by homogeneous and heterogeneous pathways. By establishing the severe limitations related to the direct catalytic synthesis of methanol from methane, we highlight the vastly superior performance of systems which produce methanol derivatives or incorporate specific measures, such as the use of multicomponent catalysts to stabilize methanol. We thereby identify methanol protection as being indispensable for future research on homogeneous and heterogeneous catalysis.
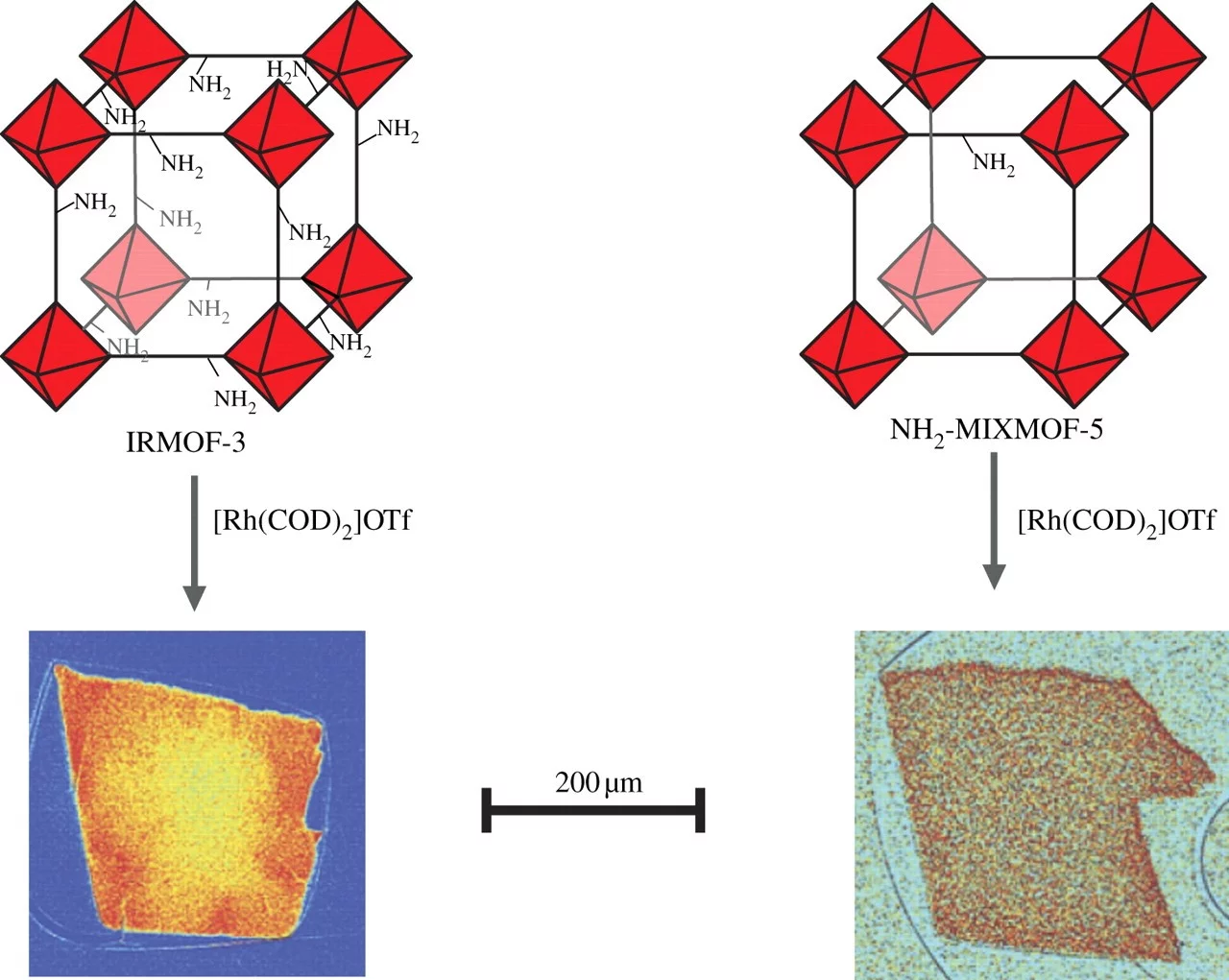
Enantioselective Hydrogenation of Olefins Enhanced by Metal–Organic Framework Additives
The use of nonprotic solvents (e.g., dichloromethane, toluene) increases the enantioselectivity of the asymmetric hydrogenation of olefins with chiral [Rh(Me‐BPE)(cod)]OTf [Me‐BPE=1,2‐bis(2,5‐dimethylphospholano)ethane; cod=1,5‐cyclooctadiene; OTf=triflate]. Readily available achiral metal–organic frameworks (MOFs) as additives yielded substantially enhanced reactivity. In toluene (but not dichloromethane), the MOFs adsorbed the homogeneous catalyst, which directly reduced rhodium contamination in the products of the reaction. The in situ formed heterogeneous catalyst was reused without loss in selectivity.
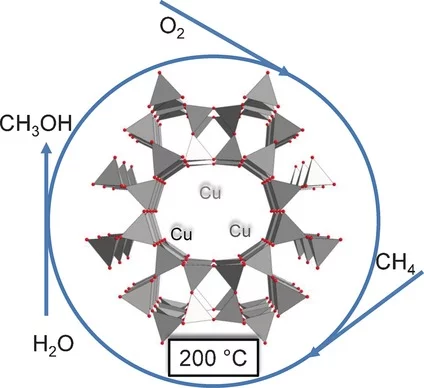
Isothermal Cyclic Conversion of Methane into Methanol over Copper‐Exchanged Zeolite at Low Temperature
Direct partial oxidation of methane into methanol is a cornerstone of catalysis. The stepped conversion of methane into methanol currently involves activation at high temperature and reaction with methane at decreased temperature, which limits applicability of the technique. The first implementation of copper‐containing zeolites in the production of methanol directly from methane is reported, using molecular oxygen under isothermal conditions at 200 °C. Copper‐exchanged zeolite is activated with oxygen, reacts with methane, and is subsequently extracted with steam in a repeated cyclic process. Methanol yield increases with methane pressure, enabling reactivity with less reactive oxidized copper species. It is possible to produce methanol over catalysts that were inactive in prior state of the art systems. Characterization of the activated catalyst at low temperature revealed that the active sites are small clusters of copper, and not necessarily di‐ or tricopper sites, indicating that catalysts can be designed with greater flexibility than formerly proposed.
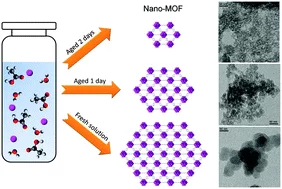
Aging of the reaction mixture as a tool to modulate the crystallite size of UiO-66 into the low nanometer range
Nanosized UiO-66 with an unprecedented crystallite size of 10 nm was synthesized by exploiting controlled aging of stock solutions of Zr4+ in N,N-dimethylformamide in the presence of variable amounts of water and acetic acid prior to the addition of the ligand. The yield of the synthesis is not affected, affording high conversion of the starting reagents into the product.
Metal organic frameworks for photo-catalytic water splitting
In an Energy & Environmental Science perspective we describe a selection of contemporary electrocatalytic, chemically promoted, and photo-catalytic investigations that apply MOFs to water oxidation and reduction half reactions. We highlight successful strategies employed by the scientific community for engineering of catalytic sites, managing redox half-reactions, introducing light sensitisation and band gap tuning – all within the confines of a framework. Major challenges toward practical application certainly remain, but the adaptive nature of MOFs stands to make a poignant contribution to the discourse on photo-catalysed water splitting.
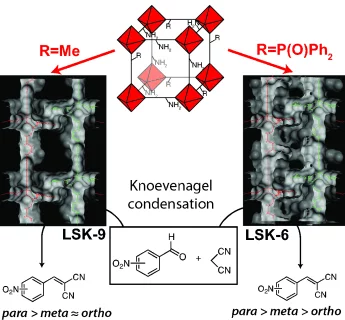
Tuning Regioisomer Reactivity in Catalysis using Bifunctional Metal–Organic Frameworks with Mixed Linkers
The activity of two bifunctional metal–organic frameworks (MOFs) with IRMOF-9 topology that contain amino, phosphine oxide and methyl groups is described. The amino group acts as an active site for the Knoevenagel condensation of ortho, meta or para nitrobenzaldehyde and malononitrile, while the non-active site diphenylphosphoryl or methyl group moderate the spatial characteristics inside the MOF pores. The functional groups induce a unique catalytic response. The reaction activity was enhanced when catalysed by MOFs comparing with aniline, whereas the phosphine oxide retards formation of the ortho isomer product.
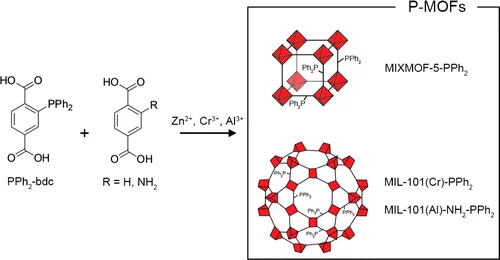
Synthesis and Characterization of Phosphine-Functionalized Metal–Organic Frameworks Based on MOF-5 and MIL-101 Topologies
Phosphine metal–organic frameworks (P-MOFs) are an emerging class of coordination polymers that provide novel opportunities for metal–supported catalysis as solid ligands. Here, we present the synthesis and characterization of three novel P-MOFs based on a (diphenylphosphino)terephthalic acid linker. We targeted the structures of MOF-5, MIL-101(Cr), and MIL-101(Al)-NH2 to produce materials with partial diphenylphosphine substitution, which were crystalline and highly porous.
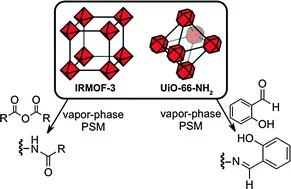
Fast and high yield post-synthetic modification of metal–organic frameworks by vapor diffusion
Vapor-phase post-synthetic modification (VP-PSM) is a tool that overcomes the limitations of standard PSM methods by giving a higher yield in short reaction times, while providing more flexibility in designing metal–organic frameworks with functionalization for chemical and physical applications. Here, we describe an efficient VP-PSM technique for PSM.
Catalytic conversion of methane to methanol over Cu–mordenite
Methane can be converted to methanol over copper-exchanged mordenite at 200 °C. Methanol could be recovered at the end of the reactor. This multi-step reaction opens the possibility for methane to methanol conversion in a closed catalytic cyclic reaction system.
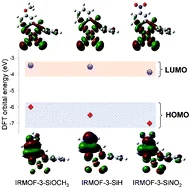
Fine tuning of gold electronic structure by IRMOF post-synthetic modification
We present a strategy to fine-tune the electronic structure of gold atoms by means of post-synthetic modification of IRMOF-3 (iso-reticular metal organic framework-3) with salicylaldehyde derivatives. Changes in the electronic structure of gold were predicted by theoretical calculations and determined by resonant inelastic X-ray scattering (RIXS) at the gold L3 edge. Modification of the coordinating group affected the relative HOMO and LUMO energy levels, resulting in a change of the HOMO–LUMO gap. The ability to fine tune electronic structure may lead to tailoring of the catalytic performance and material electronics states.
Single-atom Active Sites on Metal-organic Frameworks
Catalysts with single-atom active sites (SAHCs) allow design and fine-tuning of the active moiety, and can potentially combine the advantages of heterogeneous and homogeneous catalysis. This study illustrates how porous metal-organic frameworks (MOFs) can be synthesized with homogeneous distribution of SAHCs. The catalytic potential of MIXMOFs is shown. A short overview of catalysis with mesoporous silica materials is described to demonstrate their importance in SAHC.
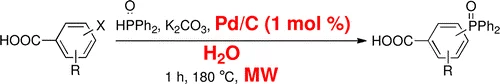
Synthesis of Water-soluble Phosphine Oxides by Pd/C-catalyzed P-C Coupling in Water
Cross-coupling between diphenylphosphine oxide and halogenated benzoic acids catalyzed by Pd/C in water is a green, simple, and fast protocol to obtain water-soluble tertiary phosphine oxides without the addition of ligands and additives. Low reaction times and microwave irradiation make this method general and excellent for laboratory and large-scale synthesis without the need to use organic solvents in reactions and workup.
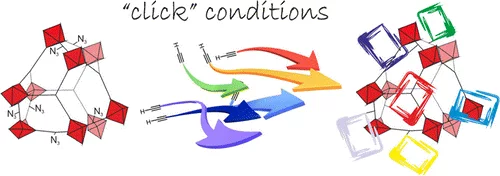
'Click' on MOFs: A Versatile Tool for the Multimodal Derivatization of N 3 -Decorated Metal Organic Frameworks
Postsynthetic modification (PSM) has been exploited for the preparation of two novel N3-decorated MOFs, namely, UMCM-1-N3 and MIXMOF-5-N3, both featuring different azido functional group content and crystalline structure. Subsequently, the as-synthesized materials were further transformed through an efficient and versatile approach capable of imparting multimodality to these porous organic–inorganic polymers. Cu-mediated acetylene–azide coupling (CuAAC; “click” reaction) has been performed in a single step, under optimized conditions, with complete azido → triazole conversion using either single acetylene derivatives or mixtures of reactive terminal alkynes in variable molar fractions to give homo- and heteroderivatized MOFs. An accurate control of the relative percentages of the functional groups in the final heterofunctionalized materials is conveniently achieved through a fine-tuning of the starting acetylene mixtures molar ratio. All MOFs derivatives have been characterized through thermogravimetric analysis coupled with MS analysis of volatiles (TG-MS), powder X-ray diffraction (PXRD), FT-IR spectroscopy, and NMR spectroscopy of the digested functionalized samples. Fluorescence studies on properly labeled (dye-functionalized) MOFs have been used for the first time to assess the statistical distribution of the “reactive probe” throughout the bulk material.
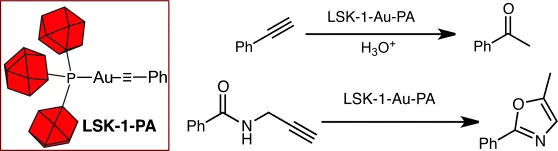
Au I Catalysis on a Coordination Polymer: A Solid Porous Ligand with Free Phosphine Sites
The best things in life are free: LSK-1 is a porous polymer with free phosphine sites homogeneously distributed within the structure. Post- functionalization gave a AuI-impregnated solid (16 % w/w) that was used as a recyclable heterogeneous catalyst.