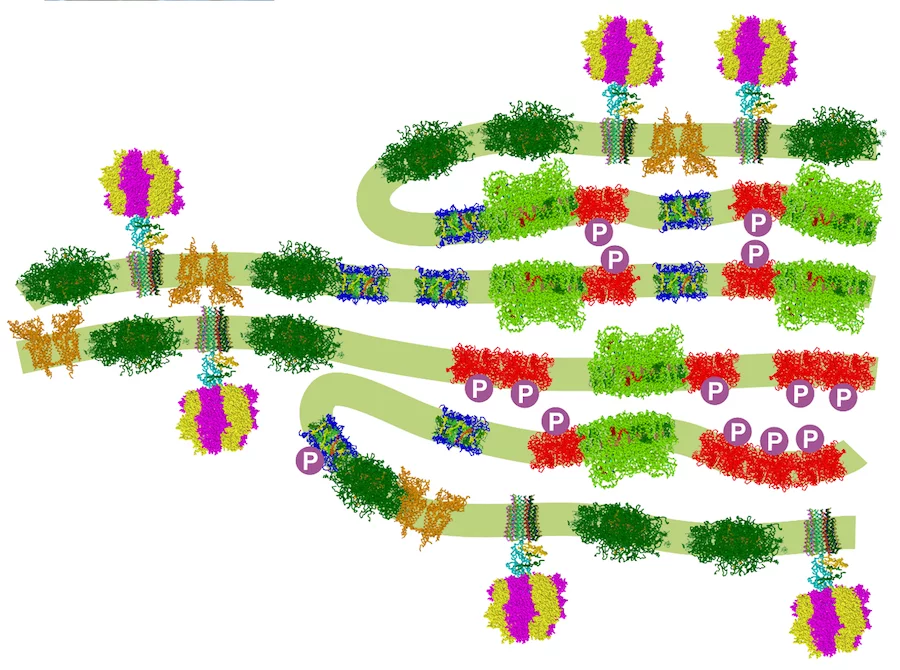
In oxygenic photosynthesis photochemical reactions occur in two different photosystems (PSs) and the light-energy conversion is regulated by balancing their activity. Such a power balance requires a sophisticated regulatory mechanism called state transitions, which involve reversible phosphorylation of the light-harvesting complex proteins (LHCIIs) to redistribute absorbed excitation energy between the two photosystems. Using noninvasive techniques (small-angle neutron scattering, circular dichroism, and absorption transient spectroscopy) in the green alga Chlamydomonas reinhardtii, we have revealed that state transitions modify the chloroplast structure, affecting the stacking and periodicity of the photosynthetic membranes and altering protein-protein interactions within these membranes. These structural changes accompany the conversion of LHCII into an energy-dissipating mode with only minor displacements of phosphorylated LHCIIs from PSII to PSI, thereby allowing us to reevaluate the physiological significance of state transitions.