A two-step thermochemical cycle for reducing CO2 into CO has been experimentally demonstrated using concentrated solar radiation as the energy source of high-temperature process heat. The first, endothermic, solar step is the thermal dissociation of zinc oxide into zinc. The second, exothermic, non-solar step is the reaction of zinc with CO2, yielding CO and the initial zinc oxide, which is recycled to the first step. A Second-Law thermodynamic analysis for the net reaction CO2=CO+0.5O2 indicates the potential of reaching solar-to-chemical energy conversion efficiencies of up to 39%. CO can be used as combustion fuel for power generation or further processed to synthetic liquid fuels for transportation.
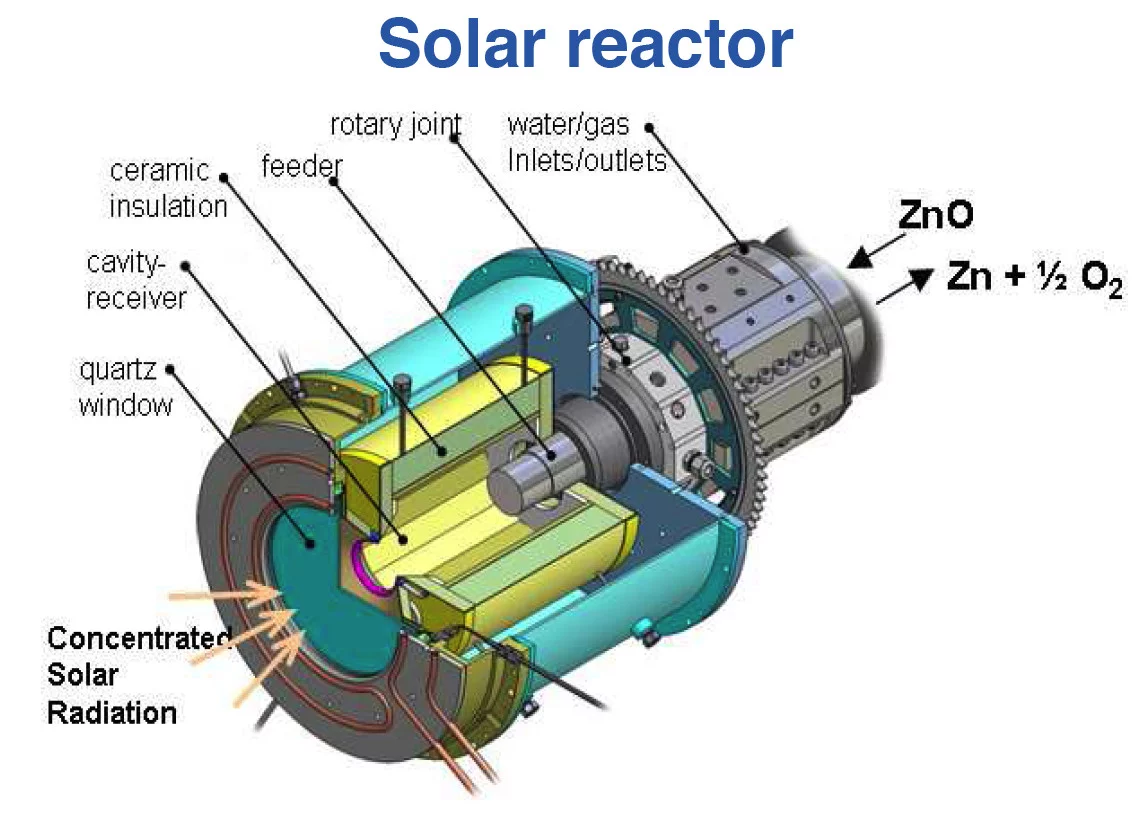
The adjacent scheme depicts the solar chemical reactor configuration for the production of Zn by ZnO-reduction at 2000 K. It features a cavity-receiver lined with ZnO particles that are directly exposed to high-flux solar irradiation, providing a very efficient heat transfer mechanism to the reaction site.
Presentation slides
The adjacent scheme depicts the solar chemical reactor configuration for the production of Zn by ZnO-reduction at 2000 K. It features a cavity-receiver lined with ZnO particles that are directly exposed to high-flux solar irradiation, providing a very efficient heat transfer mechanism to the reaction site.
Presentation slides
Citation: P. G. Loutzenhiser, M. E. Galvez, I. Hischier, A. Stamatiou, A. Frei and A. Steinfeld, energy & fuels 23 (5) 2009 2832-2839
Publication: http://dx.doi.org/10.1021/ef801142b
Further publications: LST Publications
Publication: http://dx.doi.org/10.1021/ef801142b
Further publications: LST Publications