Perovskite oxides have emerged as promising oxygen evolution reaction (OER) electrocatalysts. In this study, by combining a scalable cutting-edge synthesis method with time-resolved X-ray absorption spectroscopy (XAS) measurements, we were able to capture the dynamic local electronic and geometric structure during realistic operando conditions (i,.e., under an applied potential).
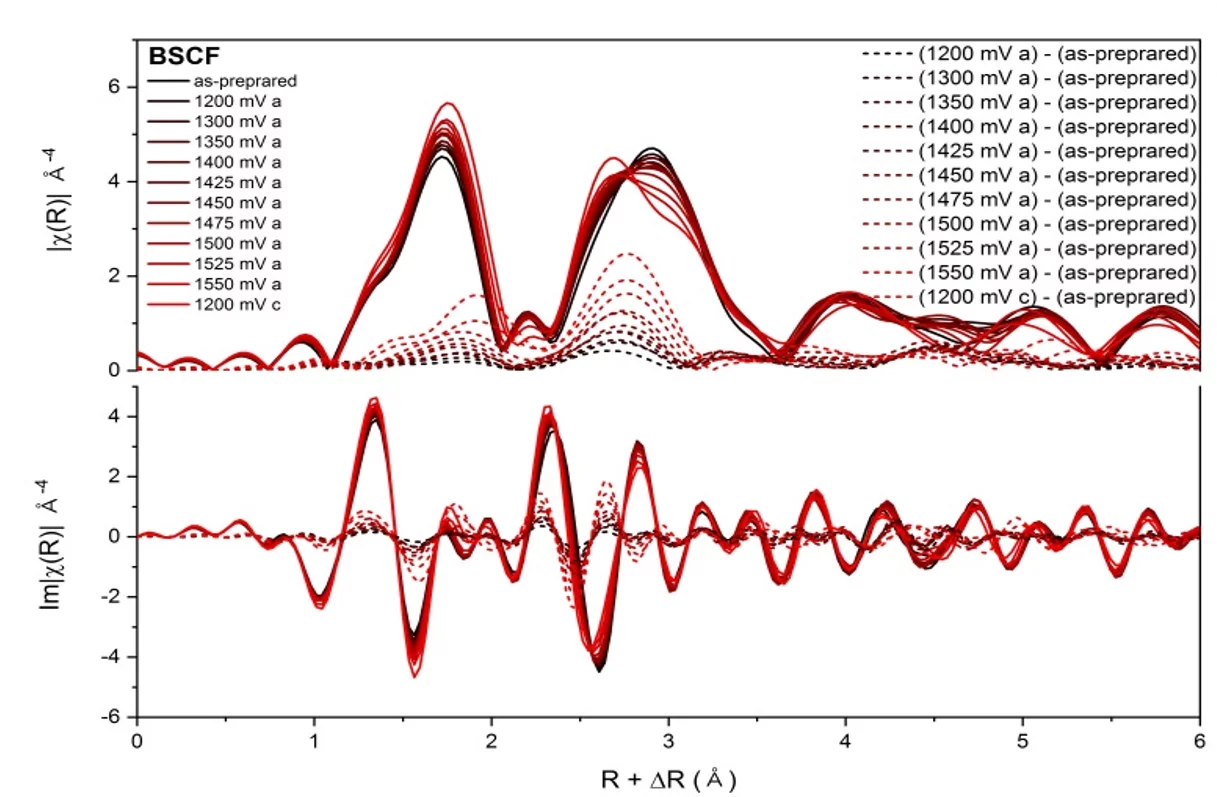
Ba0.5Sr0.5Co0.8Fe0.2O3-d (BSCF) perovskite displays unique features that allow a dynamic self-reconstruction of the material’s surface during OER, that is, the growth of a self-assembled metal oxyhydroxide active layer. In the figure operando BSCF EXAFS of XAS spectra measured at different potentials (solid lines). The dashed lines visualize the difference between individual spectrum at a certain potential and the spectrum recorded for the as prepared material.
Please refer to Nature Materials 2017, 16, 925–931 for further information.