Aerosols, suspended fine liquid or solid particles in the air we breathe, play a central role in many environmental processes through their influence on climate, the hydrological cycle, and their adverse effects on human health. It has been recently suggested that more than three million people die prematurely each year from outdoor air pollution, mainly due to aerosol particles. This is more than malaria and HIV combined, and without action the number of deaths is expected to double by 2050. While the mechanisms by which aerosol particles affect our health remain uncertain, the atmospheric oxidation of organic vapors has been shown to be related to the formation of oxygenated organic matter with high oxidative potential, the so-called reactive oxygen species (ROS). These species may damage our lung cells through oxidative stress.
Moreover, atmospheric aerosols affect Earth’s climate either directly by scattering and absorbing solar light or indirectly by acting as a base for the formation of cloud droplets, themselves important regulators of the Earth’s radiation budget. Aerosols and clouds cool the atmosphere on average; however, the magnitude of their impact remains unknown, leading to considerable uncertainties in predicting the climate sensitivity to greenhouse gases. If we want to understand the impact of human activity on our climate, we need to be able to reconstruct the conditions before the industrial era, and to determine the main ingredients responsible for the formation of aerosols and clouds. New results obtained from the cloud chamber at CERN revealed that new aerosol particles may originate from highly oxygenated organic molecules (HOMs). They are produced upon the oxidation of natural emissions and are composed of peroxides.This class of molecules seems to have important implications for climate and health.
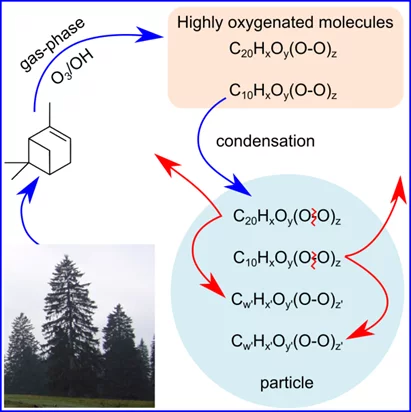
The new study by a group of researchers from the laboratory of atmospheric chemistry, at PSI, published in Chem, has revealed the fate of HOMs once they are formed from the oxidation of α-pinene, a compound emitted by trees. The researchers have used advanced mass spectrometric techniques and developed novel modelling approaches in their work. Their results suggest that these molecules, while formed very rapidly in the atmosphere, are thermodynamically unstable – i.e. they do not survive at the temperature of the ambient atmosphere (~20°C). After contributing to up to 50% of the organic aerosol mass, they decay in the aerosol phase under dark conditions, within only minutes to hours at ambient temperatures. While the decomposition of organic peroxides is well established in polymer science, used as an efficient source of free radicals for polymer initiation, these reactions have been revealed for the first time in aerosol science. It is known that organic peroxides are thermally active, and only moderately oxygenated peroxides are sufficiently stable to be produced commercially, making the rapid decomposition of HOMs not unexpected at ambient temperatures. The decomposition of these peroxide containing molecules has been found to change the properties of the organic aerosols. These reactions may have profound implications for the oxidative potential of the particle phase (e.g. radical budget), the aerosol-climate interactions and the relationship between particle characteristics and adverse health effects.
Contact
Dr Josef DommenLaboratory of Atmospheric Chemistry
Paul Scherrer Institut
Telephone: +41 56 310 29 92
E-mail: josef.dommen@psi.ch
Dr Imad El Haddad
Laboratory of Atmospheric Chemistry
Paul Scherrer Institut
Telephone: +41 56 310 29 95
E-mail: imad.el-haddad@psi.ch
Original Publication
Labile peroxides in secondary organic aerosolM. Krapf, I. El Haddad, E.A. Bruns, U. Molteni, K.R. Daellenbach, A.S.H. Prévôt, U. Baltensperger, and J. Dommen
CHEM, 2016
DOI: 10.1016/j.chempr.2016.09.007