Understanding the Interplay between Artificial SEI and Electrolyte Additives in Enhancing Silicon Electrode Performance for Li-Ion Batteries
Maintaining a stable solid electrolyte interphase (SEI) is crucial for Li-ion battery safety, especially with high-capacity anode containing silicon. Therefore, our study explored long-term cycling of Si electrodes with artificial alucone-based SEI, deposited by molecular layer deposition (MLD) in combination with a fluoroethylene carbonate (FEC) electrolyte additive. MLD of flexible Li-ion permeable artificial SEI coatings onto electrode resulted in improved capacity, enhanced Si electrode cycle life and capacity retention.
Welcome Prof. Dr. Sarbajit Banerjee as the New Head of the Laboratory for Battery Science!
We are pleased to announce that Prof. Dr. Sarbajit Banerjee has been appointed as the new Head of the PSI Laboratory for Battery Science, effective December 1, 2024. Prof. Banerjee joins us from Texas A&M University, where his internationally recognized research has advanced the fields of electrochemical energy storage and materials science.
Converting the CHF3 greenhouse gas into LiF coating for high-voltage cathode materials toward high-energy density Li-ion batteries
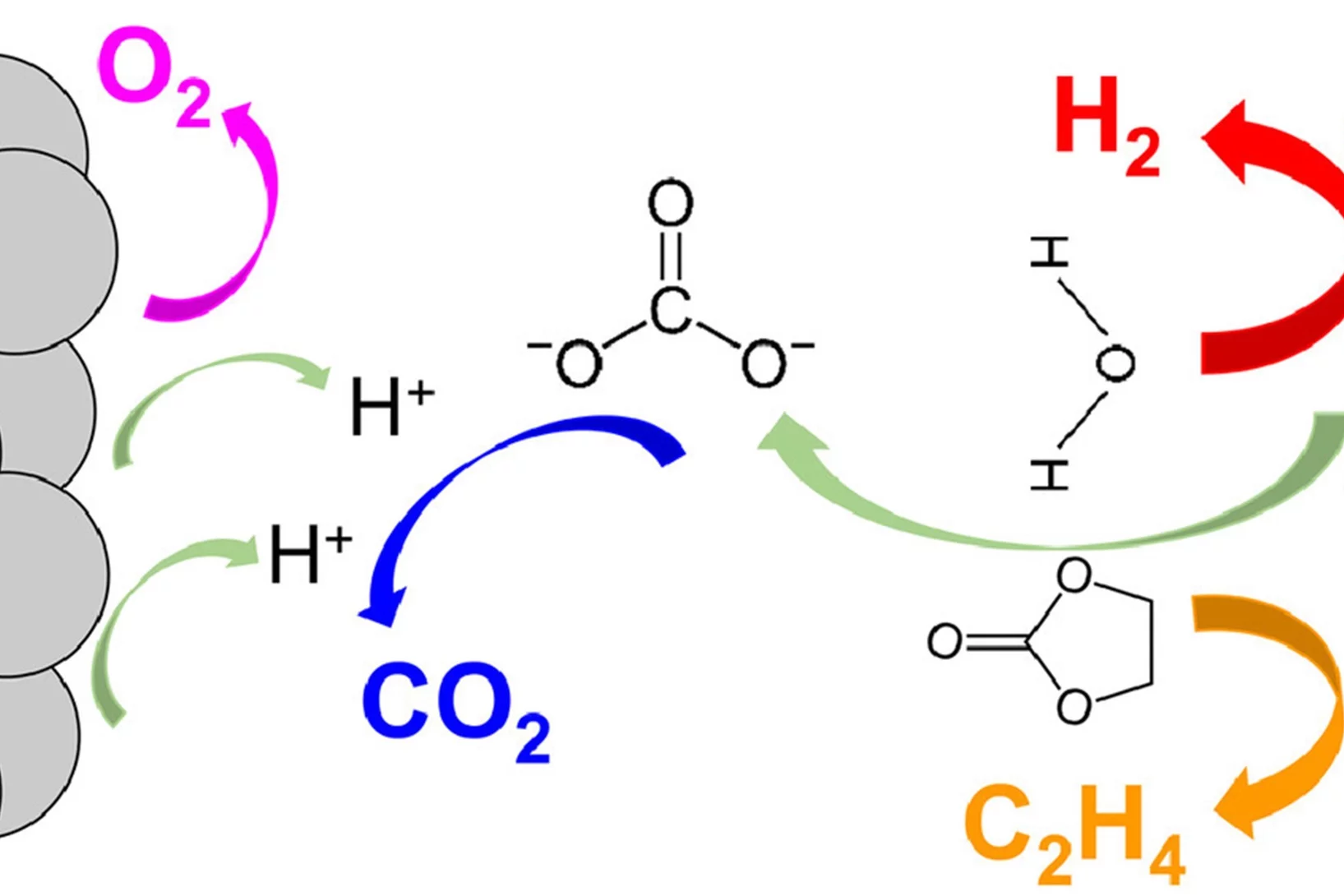
The instability and the fading of high voltage cathode materials above 4.3 V remains a major challenge for the next generation of high energy density Li-ion batteries. Here, we present a facile, environmentally friendly, cost effective and scalable method to address this problem by uniformly fluorinating the surface of cathode materials with CHF3, a mild fluorinating agent but a potent greenhouse gas. CHF3 is successfully transformed into ~2 nm LiF homogenous layer covering the surface of layered-oxide cathode materials.
Real-Time Insights into Sodium-Ion Battery Chemistry
Identification of gaseous decomposition products from irreversible side-reactions enables understanding of inner working of rechargeable batteries. Unlike for Li-ion batteries, the knowledge of the gas-evolution processes in Na-ion batteries is limited. Our study revealed that Na-ion cells develop a less stable solid-electrolyte interphase (SEI) compared to Li-ion cells due to higher solubility of SEI constituents in Na-electrolytes.
An overview about all-solid-state batteries research activities and characterization capabilities at PSI
All-solid-state batteries (ASSBs) are forecasted to play a central role in the next generation of high energy density and safe storage devices. However, ASSBs still an immature technology and require further advancements on multiple fronts like interface (electro-)chemical and mechanical instabilities. Here, we provide an overview about PSI efforts in (i) employing advanced operando laboratory and synchrotron-based analytical methods to shed light into the various degradation mechanisms and (ii) our capabilities for interface chemical engineering.
Importance of Identifying Key Experimental Parameters for the Li-ion Battery Performance Testing
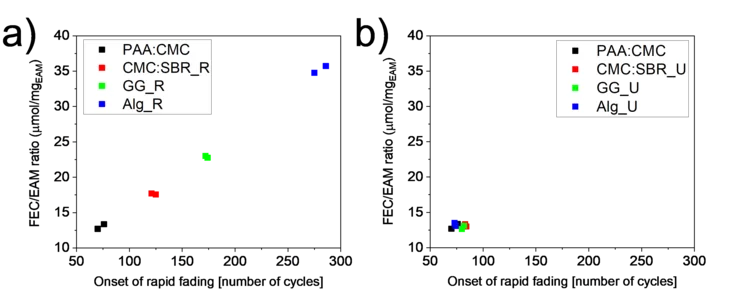
The mass loading of Si-graphite electrodes is often considered as a parameter of secondary importance when testing their performance. However, if a sacrificial additive is present in the electrolyte, the electrode loading becomes the battery cycle-life-determining factor. A lower loading was obtained by keeping slurry preparation steps unchanged from binder to binder and resulted in a longer lifetime for some of the binders. When the final loading was kept constant instead, the performance became independent of the binder used.
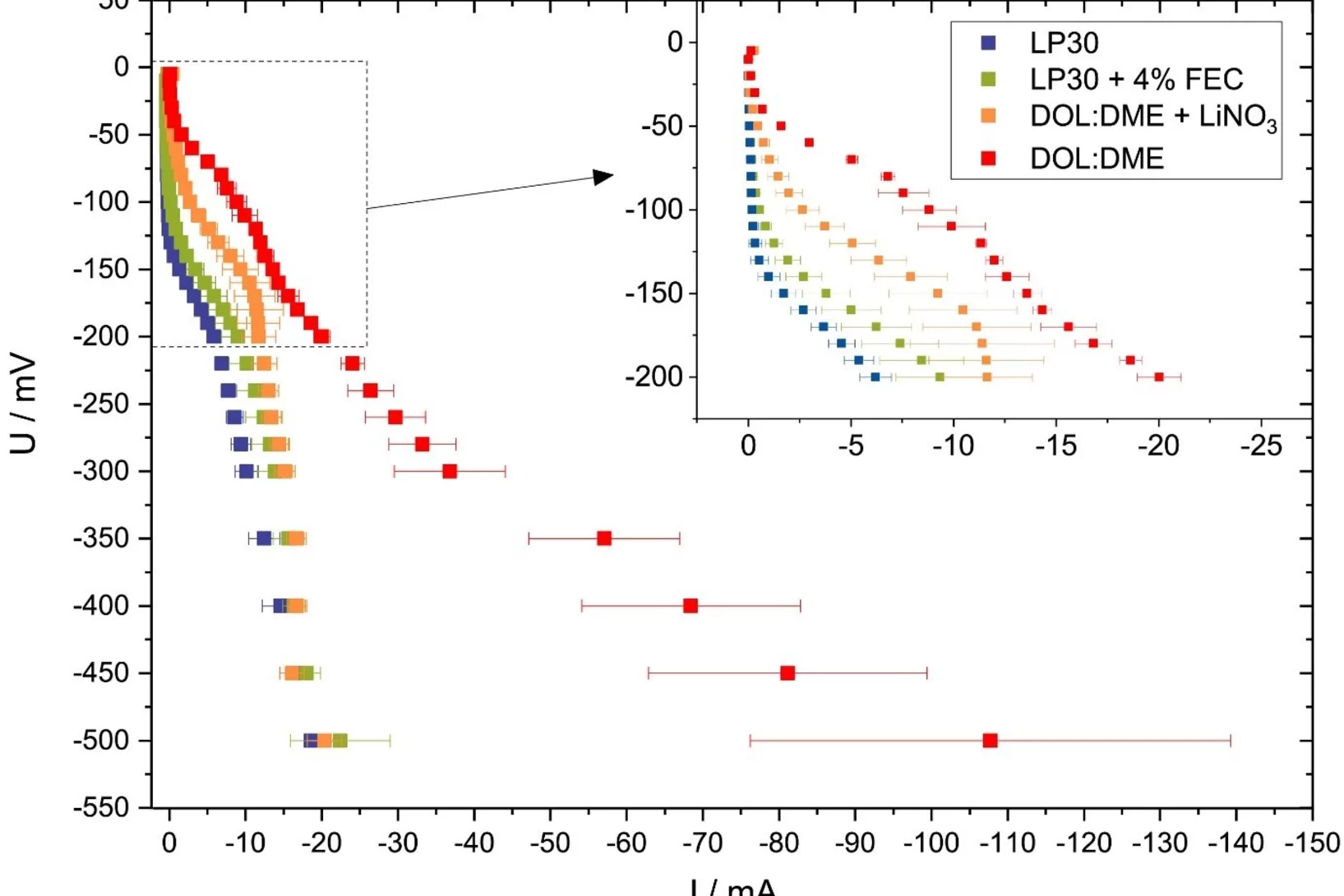
Versatile and Fast Methodology for Evaluation of Metallic Lithium Negative Battery Electrodes
Evaluating potential electrolyte candidates is typically a lengthy procedure requiring long-term cycling experiments. To speed this process up, we have investigated potentiostatic lithium plating as a potential method for fast electrolyte suitability investigation. The applications of this methodology is not limited to liquid electrolytes, - effects of solid-state electrolytes, coatings, and other modifications can be readily assessed.
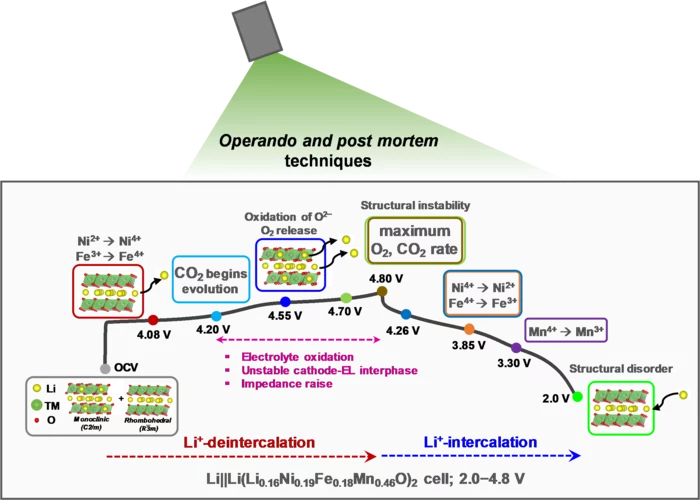
Stable Performance of High Capacity Cobalt-Free Li-ion Battery
Lithium-rich layered oxides, containing cobalt, despite being promising high-capacity cathode materials, need alternatives to eliminate toxic and geopolitically restricted cobalt. An ongoing search for low-cost, Co-free Li-rich cathode materials with a better structural stability lead to investigation of Li1.16Ni0.19Fe0.18Mn0.46O2 (LNFM), where cobalt is replaced by abundant iron. Our LNFM not only delivered a high capacity of 229 mAh/g but also has a stable average discharge voltage when cycled to upper cutoff potential of 4.8 V in additive-free electrolyte.
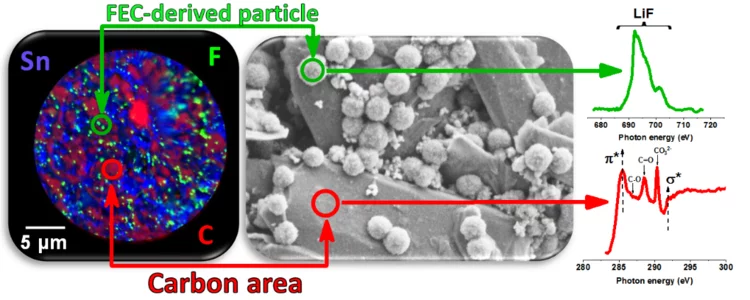
Deciphering the Mechanism of FEC-induced SEI Formation in Li-ion Batteries
Fluoroethylene-carbonate is often referred to as a film-forming electrolyte additive for Li-ion batteries, resulting in high quality Solid–Electrolyte-Interphase on negative electrode, however, the underlying mechanism, even if thought to be known, has been only clarified due to our targeted experimental design, combining systematic electrochemical, chemical and microscopy characterization techniques. We have shown that first the formation of inorganic LiF-rich particles appear and only later the carbonate-rich film is actually formed.
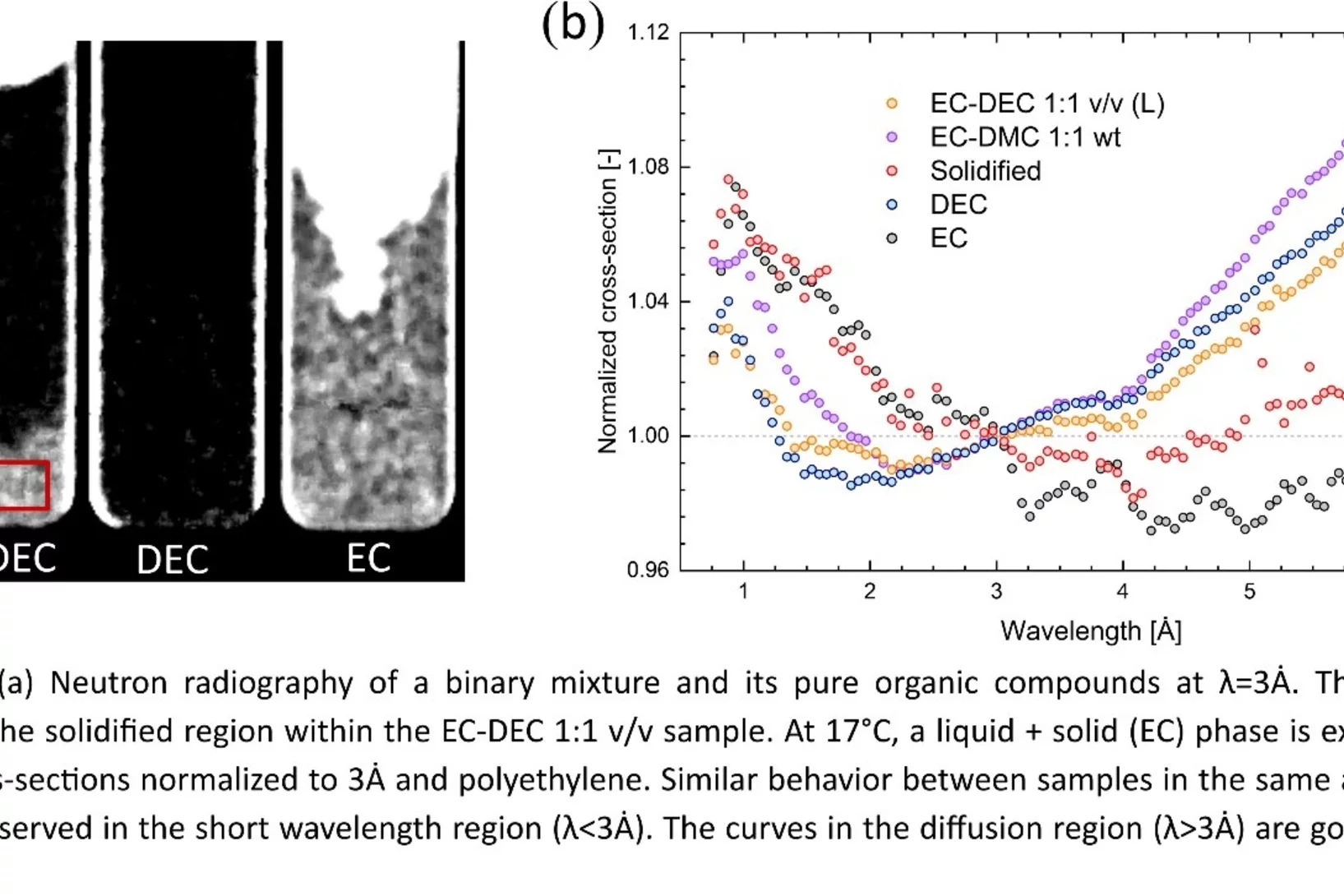
Towards in situ imaging of electrolyte physical and chemical changes in Li-ion batteries
The Lithium conducting electrolyte is a critical component of Li-ion batteries, as it has an important impact on performance and its durability, in particular in case of extreme environmental conditions. At low temperatures, a partial solidification can occur, while high temperatures can promote the degradation of the electrolyte. Using time-of-flight (ToF) neutron radiography, the possibility of imaging such changes in a fully non-invasive manner was demonstrated for the first time.
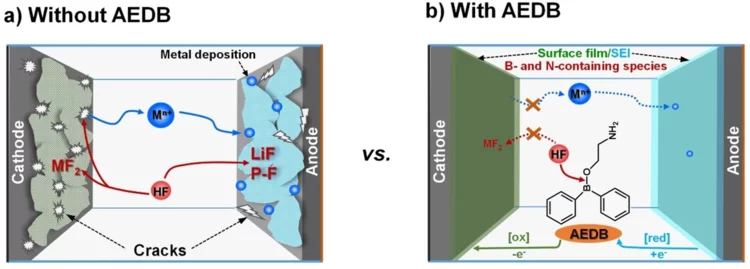
Cross-Talk–Suppressing Electrolyte Additive for Li-ion Batteries
Control of interfacial reactivity at high-voltage is a key to high-energy-density Li-ion batteries. 2-aminoethyldiphenyl borate was investigated as an electrolyte additive to stabilize surface and bulk of both NCM851005 and graphite in the cell with upper cut-off voltage of 4.4 V vs Li+/Li. AEDB almost completely eliminated the “cross-talk” in the cell, by significantly reducing metal leaching from the cathode, preventing their deposition at the anode, and further electrolyte decomposition.
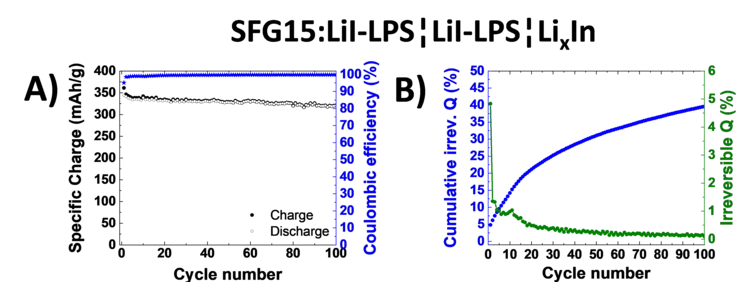
Excellent cycling stability of graphite in all-solid-state battery using sulfide solid electrolyte
All-solid-state lithium ion batteries represent a promising battery technology for boosting the volumetric energy density and promising a superior safety. In this study excellent cycling stability of graphite anode material have been demonstrated in combination with sulfide-based solid electrolyte. Furthermore we evaluated the stability of the graphite-electrolyte interface by analyzing the normalized cumulative irreversible charge during cycling experiments.
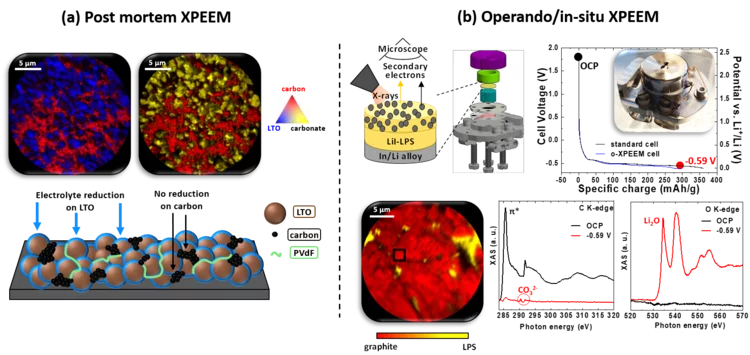
Post mortem/operando XPEEM: for studying the surface of single particle in Li-ion battery electrodes
X-ray photoemission electron microscopy (XPEEM) with its excellent spatial resolution is a well-suited technique to elucidate the complex electrode-electrolyte interface reactions in Li-ion batteries. It provides element-specific contrast images and enables the acquisition of local X-ray absorption spectra on single particles. Here we demonstrate the strength of post mortem measurements and we show the first electrochemical cell dedicated for operando experiments in all-solid-state batteries.
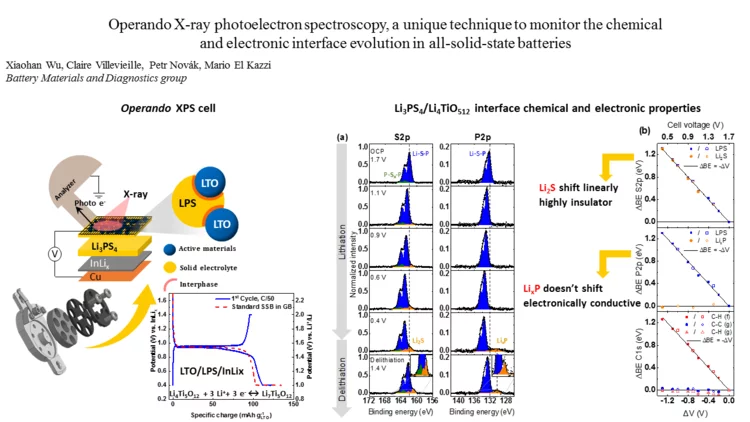
Operando X-ray photoelectron spectroscopy, to monitor the chemical and electronic interface evolution in all-solid-state batteries
Degradation of the solid-electrolyte interface occurring during cycling is currently one of the most challenging issues for the development of all-solid-state batteries. Here we designed a unique electrochemical custom made cell for operando X-ray photoelectron spectroscopy (XPS) capable of maintaining high mechanical pressure with reliable electrochemistry and able to monitor in real-time the surface (electro-)chemical reactivity at the interfaces between the different composite.
Dosing Differential Electrochemical Mass Spectrometry (D-DEMS) for Li-O2 Batteries
The high-energy rechargeable Li-O2 battery has been subject to intensive research worldwide during the past years. The Li-O2 cell mainly comprises a negative (e.g. Li metal) and positive (e.g. porous carbon) electrode separated by an electronically insulating, but Li+ conducting electrolyte layer. In order to study the cell chemistry, a differential electrochemical mass spectrometry setup based on a set of valves, a pressure sensor and a quadrupole mass spectrometer has been developed.