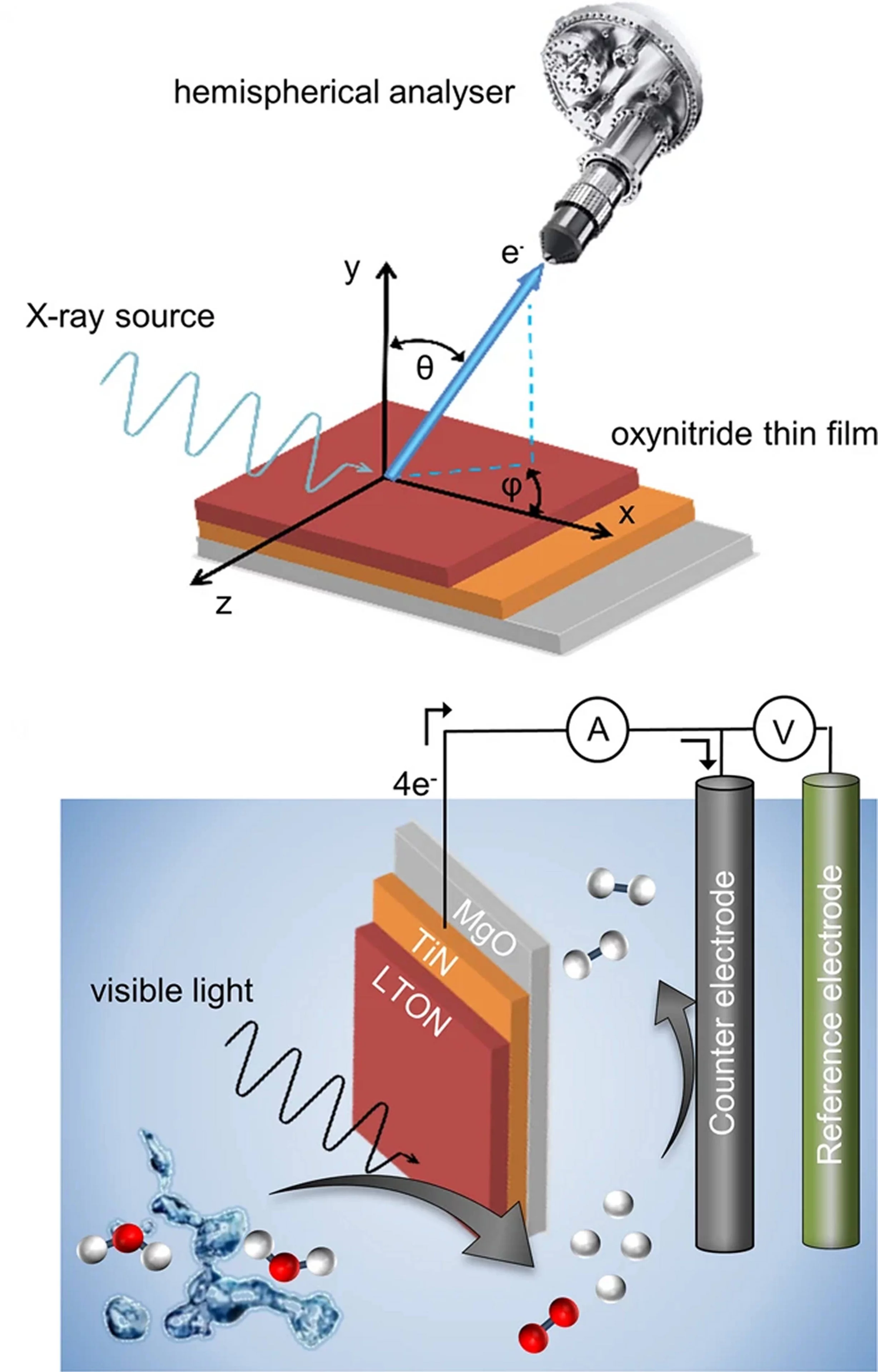
Oxynitrides are promising materials for visible light-driven water splitting. However, limited information regarding their electron-momentum resolved electronic structure exists. Here, with the advantage of the enhanced probing depth and chemical state specificity of soft-X-ray ARPES, we determine the electronic structure of the photocatalyst oxynitride LaTiO2N and monitor its evolution as a consequence of the oxygen evolution reaction. After the photoelectrochemical reactions, we observe a partial loss of Ti- and La-N 2p states, distortions surrounding the local environment of titanium atoms and, unexpectedly, an indication of an electron accumulation layer at or near the surface, which may be connected with either a large density of metallic surface states or downward band bending. The distortions and defects associated with the titanium 3d states lead to the trapping of electrons and charge recombination, which is a major limitation for the oxynitride LaTiO2N. The presence of an accumulation layer and its evolution suggests complex mechanisms of the photoelectrochemical reaction, especially in cases where co-catalysts or passivation layers are used.
Reference: C. Lawley et al. , Commun Mater 4, 15 (2023)
Read full article: here