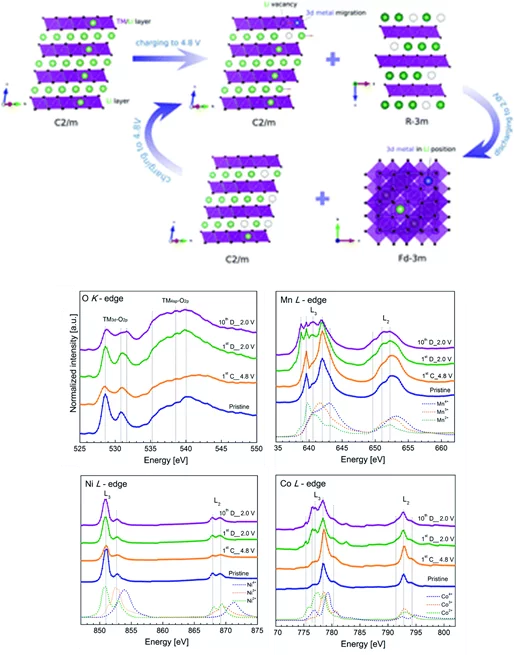
New generation of cathode materials from the Li-rich NMC group are under constant investigation due to their extremely high energy densities resulting from redox reactions involving both transition metals and lattice oxygen. Although a lot of research has been taken so far, the exact mechanism of lithium (de)insertion in those materials, especially the reactions involving redox of lattice oxygen is still elusive. Due to the design of the batteries the observed reactions starts at the surface of the electrode which contacts the electrolyte and as reaction continues, goes deeper into the bulk structure. In order to follow the reactions taking place in Li-rich NMC materials we aimed to exactly distinguish and characterize the phase transitions taking place on the surface and within the bulk of the Li1.2Mn0.6Ni0.1Co0.1O2 electrode. To do so we used comprehensive XAS measurements performed at PHOENIX beamline. The use of both TEY (Total Electron Yield) and TFY (Total Fluorescence Yield) modes, probing surface and the bulk respectively, helped us to characterize and spatially distinguish the phases present in the materials at different state of charge. Investigation of the transition metal L-edges (Co, Ni, Mn) and the oxygen K-edge provided us a unique insight into the contribution of each element into the electrochemical reactions taking place in Li-rich NMC type materials. We found that during the battery lifetime (more than 100 cycles), the Li1.2Mn0.6Ni0.1Co0.1O2 electrode undergoes several phase transition including a reversible superstructure formation accompanied by the disappearance of the rhombohedral phase and the development of the spinel structure on the surface of the electrode. Thanks to the XAS spectra obtained at the PHOENIX beamline, we were able to shed light on the spatial evolution of the crystal structure of Li1.2Mn0.6Ni0.1Co0.1O2 and explain in what manner the transition metals and oxygen atoms take part in the electrochemical reactions.
Contact
Energy and Environment Division, OLGA 117
Paul Scherrer Institut
Telephone: +41 56 310 2457
E-mail: petr.novak@psi.ch
Dr. Camelia Borca
Swiss Light Source
Paul Scherrer Institut
Telephone: +41 56 310 5478
The impact of oxygen evolution and cation migration on the cycling stability of a Li-rich Li[Li0.2Mn0.6Ni0.1Co0.1]O2 positive electrode
K. Redel, A. Kulka, K. Walczak, A. Plewa, C.N. Borca and J. Molenda
Journal of Materials Chemistry A, 2020, 8(35), 18143-18153
DOI: 10.1039/D0TA05556E