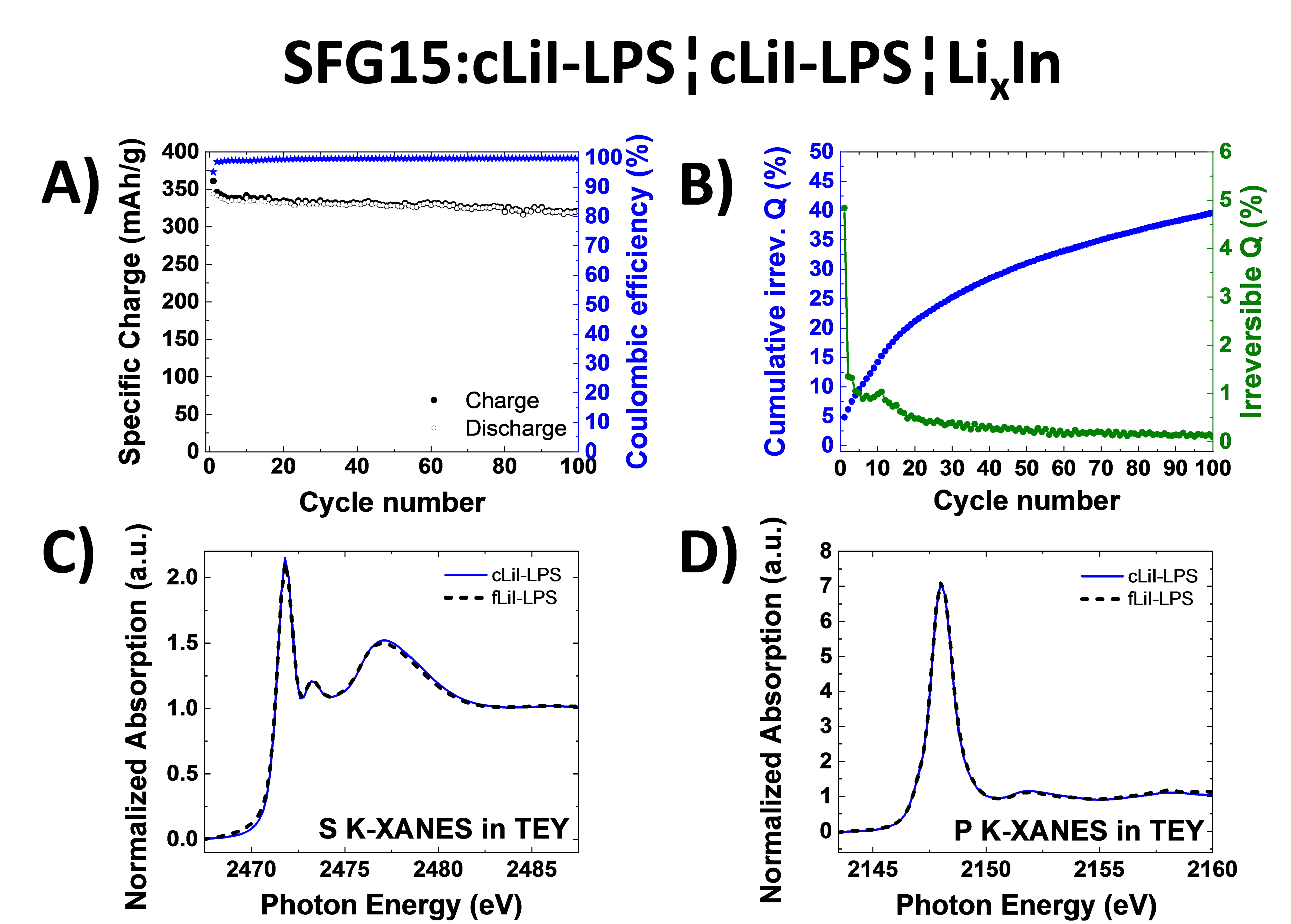
Graphite has been commercialized as negative electrode material for Li-ion batteries for almost three decades. Its interfacial stability with conventional liquid electrolytes has already been thoroughly investigated. As one of the next-generation lithium-ion battery technologies, the so-called all-solid-state batteries are proposed due their inherent higher temperature stability and their potential capability to reach higher energy densities.
Sulfide-based solid electrolytes are particularly attractive as they present high ionic conductivity and can be easily processed at room temperature. For this study, two different solid electrolytes 0.75Li2S-0.25P2S5 (LPS) and 0.3LiI-0.7(0.75Li2S-0.25P2S5) were tested in combination with graphite and the stability of the graphite-solid electrolyte interface was evaluated by analyzing the normalized cumulative irreversible charge. The electrochemical performance of graphite with different particle size and morphology were reported with respectively fine (< 1 µm) and coarse (5 µm range) solid electrolytes. The solid electrolytes were characterized via electrochemical impedance spectroscopy, X-ray diffraction, Raman spectroscopy and finally via X-ray absorption spectroscopy (XAS). The XAS spectra were recorded at the PHOENIX beamline in Total Electron Yield (TEY) mode, which is surface sensitive. To verify that the coarse electrolyte after having been exposed to a wet pulverization step doesn’t present any sulfur and phosphorus local environment changes, the measurements were performed at the S and P K-edges.
By decreasing the particle size of the solid electrolyte, the utilization of the graphite electrode can be improved but the interfacial degradation worsens as the cumulative irreversible charge per cycle is higher. Besides, the addition of LiI didn’t improve the irreversible charge consumption but helped to deliver higher specific charges at low C-rates.
Contact
Prof. Dr. Petr Novak
Energy and Environment Division, OLGA 117
Paul Scherrer Institut
Telephone: +41 56 310 2457
E-mail: petr.novak@psi.ch
Dr. Mario El Kazzi
Energy and Environment Division, OVGA 118
Paul Scherrer Institut
Telephone: +41 56 310 5149
E-mail: mario.elkazzi@psi.ch
Dr. Thomas Huthwelker
Swiss Light Source
Paul Scherrer Institut
Telephone: +41 56 310 5314
E-mail: thomas.huthwelker@psi.ch
Original Publication
Study of Graphite Cycling in Sulfide Solid Electrolytes
Laura Höltschi, Franziska Jud, Camelia Borca, Thomas Huthwelker, Claire Villevieille, Vincent Pelé, Christian Jordy, Mario El Kazzi and Petr Novák, J. Electrochem. Soc., 2020, 167, 110558