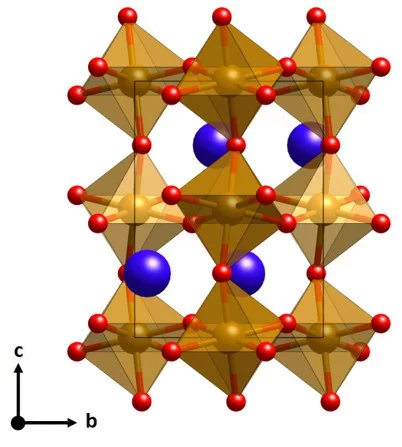
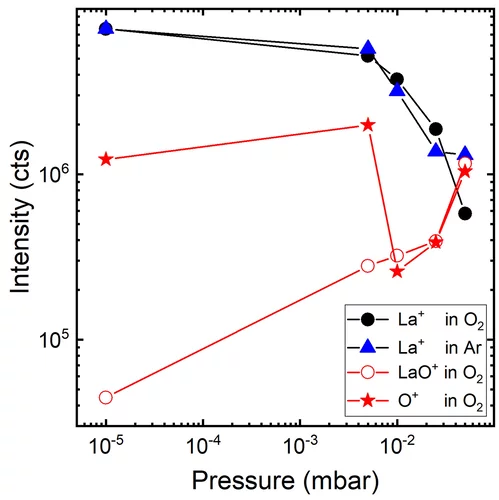
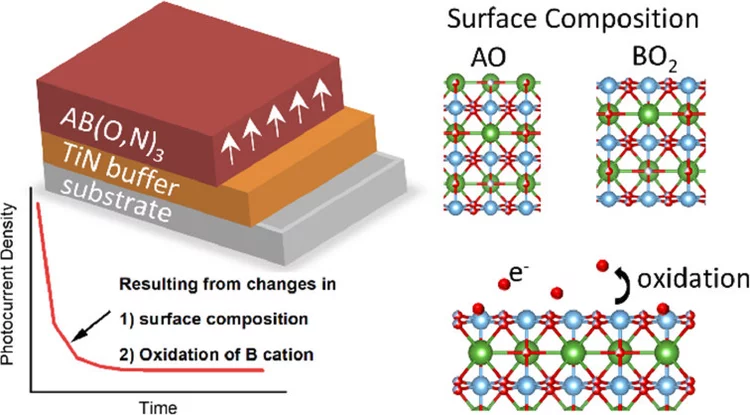
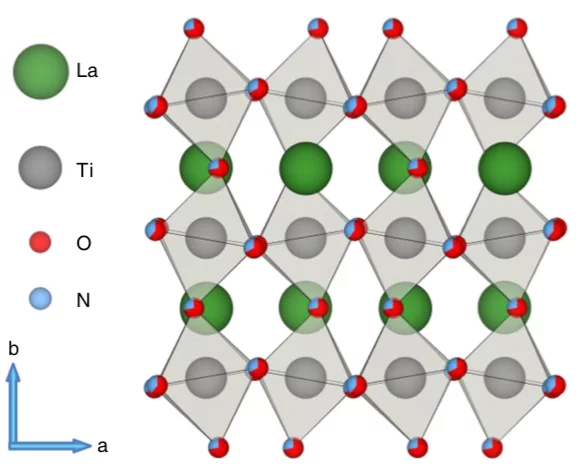
Anionic Disorder and Its Impact on the Surface Electronic Structure of Oxynitride Photoactive Semiconductors
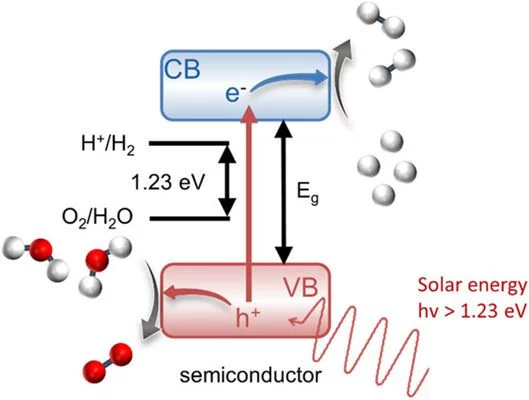
The conversion of solar energy into chemical energy, stored in the form of hydrogen, bears enormous potential as a sustainable fuel for powering emerging technologies. Photoactive oxynitrides are promising materials for splitting water into molecular oxygen and hydrogen. However, one of the issues limiting widespread commercial use of oxynitrides is degradation during operation. While recent studies have shown the loss of nitrogen, its relation to reduced efficiency has not been directly and systematically addressed with experiments. In this study, we demonstrate the impact of the anionic stoichiometry of BaTaOxNy on its electronic structure and functional properties. Through experimental ion scattering, electron microscopy, and photoelectron spectroscopy investigations, we determine the anionic composition ranging from the bulk toward the surface of BaTaOxNy thin films. This further serves as input for band structure computations modeling the substitutional disorder of the anion sites. Combining our experimental and computational approaches, we reveal the depth-dependent elemental composition of oxynitride films, resulting in downward band bending and the loss of semiconducting character toward the surface. Extending beyond idealized systems, we demonstrate the relation between the electronic properties of real oxynitride photoanodes and their performance, providing guidelines for engineering highly efficient photoelectrodes and photocatalysts for clean hydrogen production.
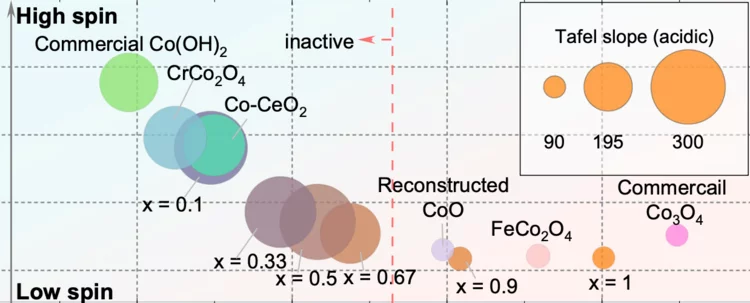
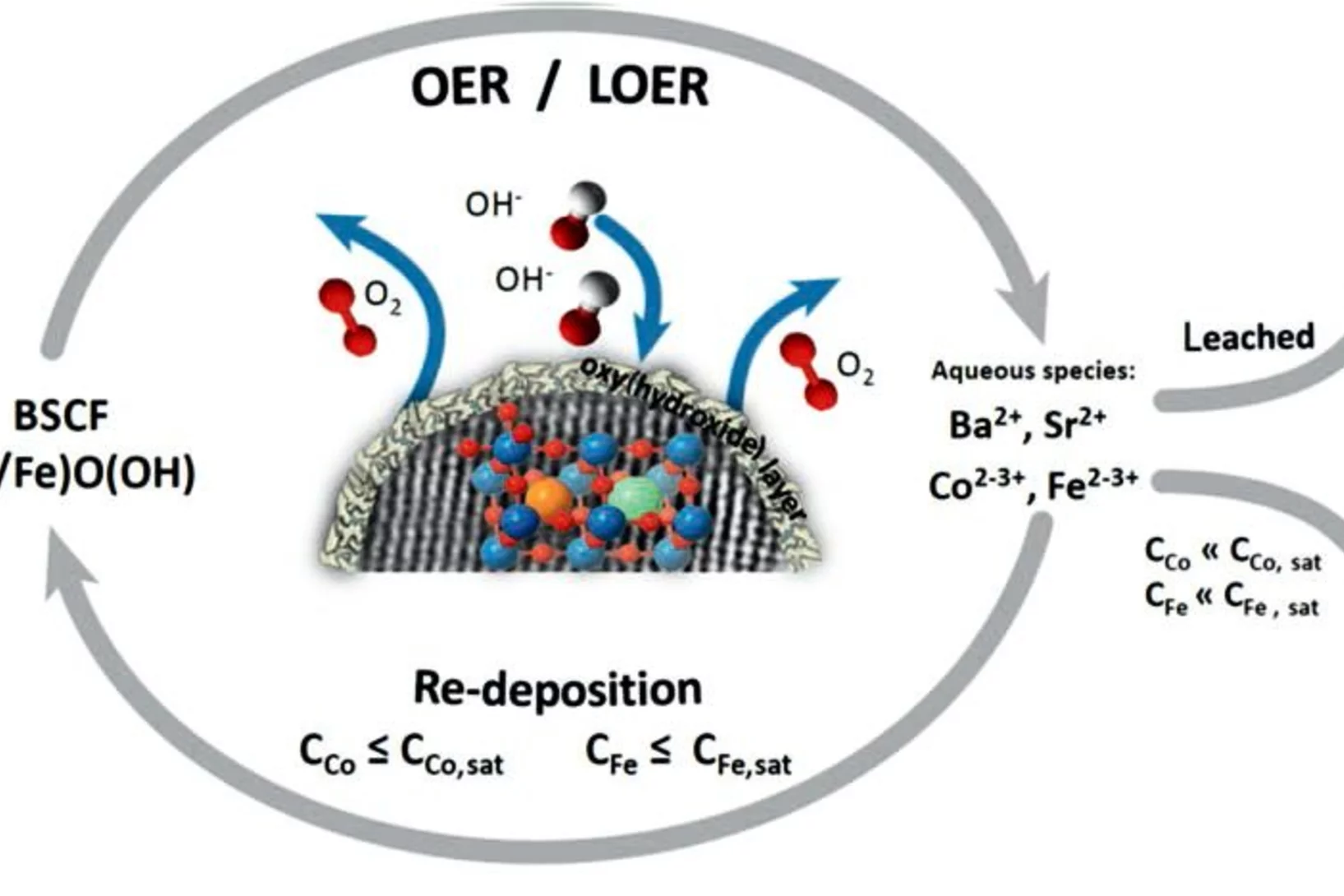
Surface oxidation/spin state determines oxygen evolution reaction activity of cobalt-based catalysts in acidic environment
Co-based catalysts are promising candidates to replace Ir/Ru-based oxides for oxygen evolution reaction (OER) catalysis in an acidic environment. However, both the reaction mechanism and the active species under acidic conditions remain unclear. In this study, by combining surface-sensitive soft X-ray absorption spectroscopy characterization with electrochemical analysis, we discover that the acidic OER activity of Co-based catalysts are determined by their surface oxidation/spin state.
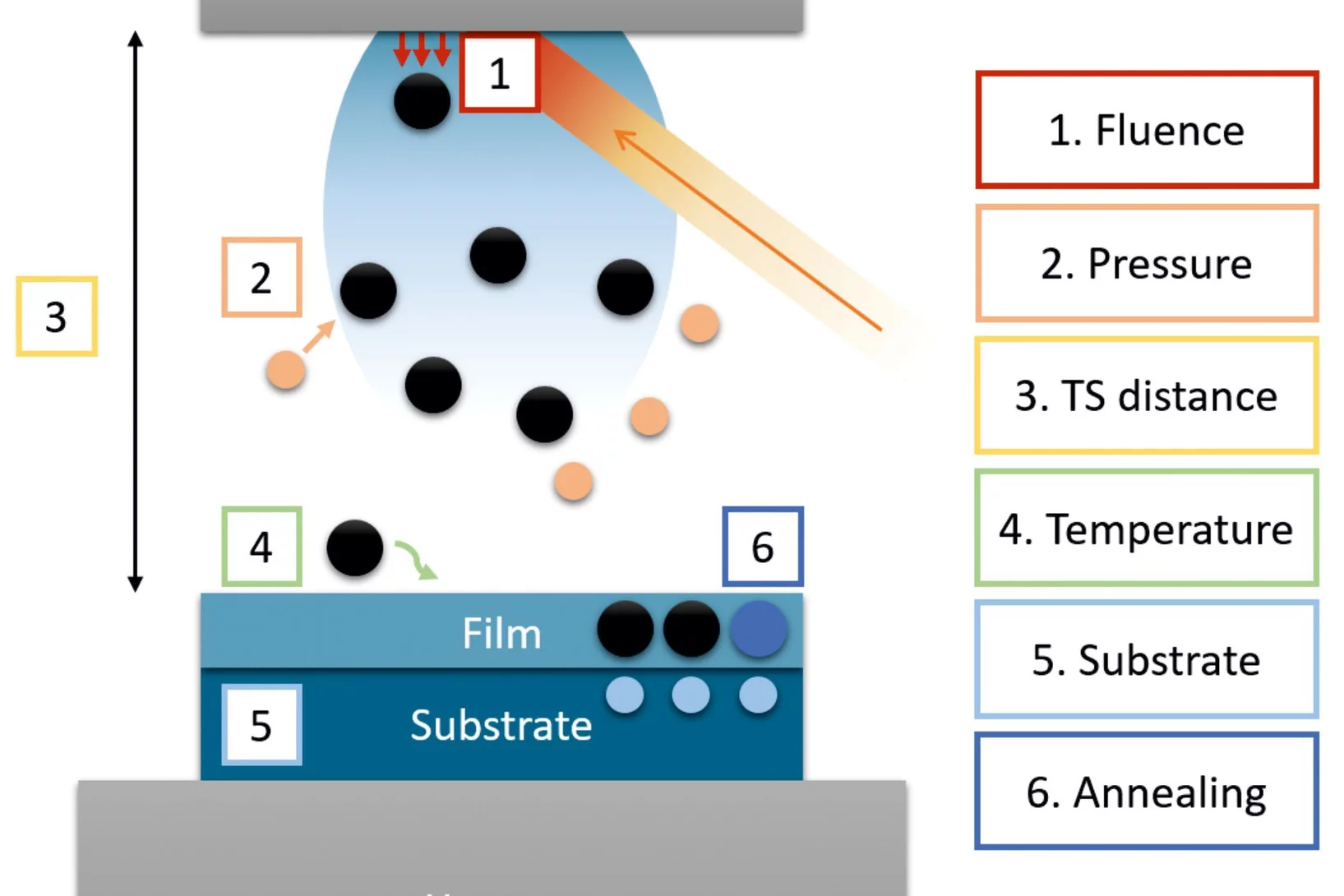
A practical guide to pulsed laser deposition
Nanoscale thin films are widely implemented across a plethora of technological and scientific areas, and form the basis for many advancements that have driven human progress, owing to the high degree of functional tunability based on the chemical composition. Pulsed laser deposition is one of the multiple physical vapour deposition routes to fabricate thin films, employing laser energy to eject material from a target in the form of a plasma ...
Momentum-resolved electronic structure of LaTiO2N photocatalysts by resonant Soft-X-ray ARPES
Oxynitrides are promising materials for visible light-driven water splitting. However, limited information regarding their electron-momentum resolved electronic structure exists. Here, with the advantage of the enhanced probing depth and chemical state specificity of soft-X-ray ARPES, we determine the electronic structure of the photocatalyst oxynitride LaTiO2N and monitor its evolution as a consequence of the oxygen evolution reaction. After the photoelectrochemical reactions, we observe a partial loss of Ti- and La-N 2p states, distortions surrounding the local environment of titanium atoms and, unexpectedly, an indication of an electron accumulation layer at or near the surface, which may be connected with either a large density of metallic surface states or downward band bending. The distortions and defects associated with the titanium 3d states lead to the trapping of electrons and charge recombination, which is a major limitation for the oxynitride LaTiO2N. The presence of an accumulation layer and its evolution suggests complex mechanisms of the photoelectrochemical reaction, especially in cases where co-catalysts or passivation layers are used.
PLD plasma plume analysis, a summary of the PSI contribution
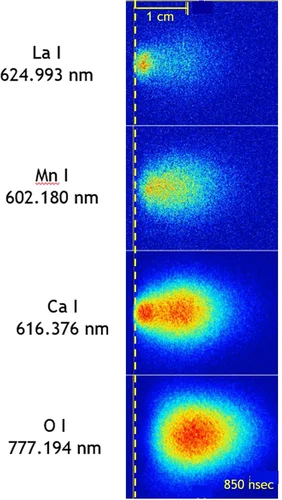
We report on the properties of laser-induced plasma plumes generated by ns pulsed excimer lasers as used for pulsed laser deposition to prepare thin oxide films. A focus is on the time and spatial evolution of chemical species in the plasma plume as well as the mechanisms related to the plume expansion. The overall dynamics of such a plume is governed by the species composition in particular if three or more elements are involved. We studied the temporal evolution of the plume, the composition of the chemical species in the plasma, as well as their electric charge. In particular, ionized species can have an important influence on film growth. Likewise, the different oxygen sources contributing to the overall oxygen content of an oxide film are presented and discussed. Important for the growth of oxide thin films is the compositional transfer of light element such as oxygen or Li. We will show and discuss how to monitor these light elements using plasma spectroscopy and plasma imaging and outline some consequences of our experimental results.
Thin-Film Oxynitride Photocatalysts for Solar Hydrogen Generation: Separating Surface and Bulk Effects Using Synchrotron X-Ray and Neutron-Based Techniques
The conversion of solar light into hydrogen by photoelectrochemical water splitting is one of the potential strategies that can allow the development of a carbon-neutral energy cycle. Oxynitride semiconductors are promising materials for this application, although important limitations must still to be addressed. One of the most important issues is physicochemical degradation of the semiconductor, at the interface with water, where the electrochemical reactions occur. In this regard, thin films, with well-defined and atomically flat surfaces, are invaluable tools for characterizing material properties and degradation mechanisms, while identifying strategies to mitigate detrimental effects. Thin oxynitride films may allow the use of complementary characterizations, not applicable to conventional powder samples. In particular, the study of the solid–liquid interface can benefit enormously from the use of thin films for synchrotron-based surface-sensitive X-Ray scattering methods and neutron reflectometry. These investigation approaches promise to speed up the design and discovery of new materials for the production of solar fuels, while paving the way for similar applications in other research fields. This work aims at reviewing the literature contributions on oxynitride thin films for solar water splitting summarizing what is learnt so far and suggesting experimental strategies to unveil what is still not clear.
Role of Dy on the magnetic properties of orthorhombic DyFeO3
Orthoferrites are a class of magnetic materials with a magnetic ordering temperature above 600 K, predominant G-type antiferromagnetic ordering of the Fe-spin system and, depending on the rare-earth ion, a spin reorientation of the Fe spin taking place at lower temperatures. DyFeO3 is of particular interest since the spin reorientation is classified as a Morin transition with the transition temperature depending strongly on the Dy-Fe interaction. Here, we report a detailed study of the magnetic and structural properties of microcrystalline DyFeO3 powder and bulk single crystal using neutron diffraction and magnetometry between 1.5 and 450 K. We find that, while the magnetic properties of the single crystal are largely as expected, the powder shows strongly modified magnetic properties, including a modified spin reorientation and a smaller Dy-Fe interaction energy of the order of 10 μeV. Subtle structural differences between powder and single crystal show that they belong to distinct magnetic space groups. In addition, the Dy ordering at 2 K in the powder is incommensurate, with a modulation vector of 0.0173(5) c∗, corresponding to a periodicity of ∼58 unit cells.
New Insight into the Gas Phase Reaction Dynamics in Pulsed Laser Deposition of Multi-Elemental Oxides
The gas-phase reaction dynamics and kinetics in a laser induced plasma are very much dependent on the interactions of the evaporated target material and the background gas. For metal (M) and metal–oxygen (MO) species ablated in an Ar and O2 background, the expansion dynamics in O2 are similar to the expansion dynamics in Ar for M+ ions with an MO+ dissociation energy smaller than O2. This is different for metal ions with an MO+ dissociation energy larger than for O2. This study shows that the plume expansion in O2 differentiates itself from the expansion in Ar due to the formation of MO+ species. It also shows that at a high oxygen background pressure, the preferred kinetic energy range to form MO species as a result of chemical reactions in an expanding plasma, is up to 5 eV.
Reconfigurable halide perovskite nanocrystal memristors for neuromorphic computing
Many in-memory computing frameworks demand electronic devices with specific switching characteristics to achieve the desired level of computational complexity. Existing memristive devices cannot be reconfigured to meet the diverse volatile and non-volatile switching requirements, and hence rely on tailored material designs specific to the targeted application, limiting their universality. “Reconfigurable memristors” that combine both ionic diffusive and drift mechanisms could address these limitations, but they remain elusive. Here we present a reconfigurable halide perovskite nanocrystal memristor that achieves on-demand switching between diffusive/volatile and drift/non-volatile modes by controllable electrochemical reactions.
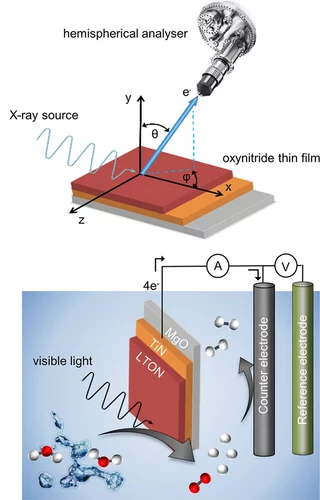
Surface Analysis of Perovskite Oxynitride Thin Films as Photoelectrodes for Solar Water Splitting
Perovskite oxynitride semiconductors have attracted huge interest recently as promising photoelectrode materials for photoelectrochemical (PEC) water splitting. Oxynitride thin films grown by physical vapor deposition are ideal model systems to study the fundamental physical and chemical properties of the surface of these materials, including their evolution. Using a combination of high-sensitivity low-energy ion scattering (LEIS) and X-ray photoelectron spectroscopy (XPS), the surface evolution of LaTiOxNy (LTON) and CaNbOxNy (CNON) thin films before and after the PEC characterizations is monitored. This work provides therefore insight into the surface characteristics and evolution of LTON and CNON oxynitride thin films as photoelectrodes for PEC applications.
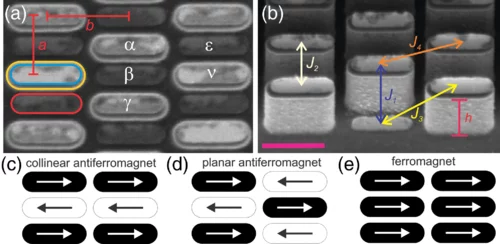
Geometrical Frustration and Planar Triangular Antiferromagnetism in Quasi-Three-Dimensional Artificial Spin Architecture
We present a realization of highly frustrated planar triangular antiferromagnetism achieved in a quasi-three-dimensional artificial spin system consisting of monodomain Ising-type nanomagnets lithographically arranged onto a deep-etched silicon substrate. We demonstrate how the three-dimensional spin architecture results in the first direct observation of long-range ordered planar triangular antiferromagnetism, in addition to a highly disordered phase with short-range correlations, once competing interactions are perfectly tuned. Our work demonstrates how escaping two-dimensional restrictions can lead to new types of magnetically frustrated metamaterials.
Pulsed Laser Deposition as a Tool for the Development of All Solid-State Microbatteries
All-solid-state lithium ion batteries (LIB) are currently the most promising technology for next generation electrochemical energy storage. Many efforts have been devoted in the past years to improve performance and safety of these devices. Nevertheless, issues regarding chemical and mechanical stability of the different components still hinder substantial improvements. Pulsed laser deposition (PLD) has proved to be an outstanding technique for the deposition of thin films of materials of interest for the fabrication of LIB. Thanks to its versatility and possible fine tuning of the thin film properties, PLD promises to be a very powerful tool for the fabrication of model systems which would allow to study in detail material properties and mechanisms contributing to LIB degradation. Nevertheless, PLD presents difficulties in the deposition of LIB components, mainly due to the presence of elements with large difference of atomic mass in their chemical composition. In this review, we report the main challenges and solution strategies used for the deposition through PLD of complex oxides thin films for LIB.
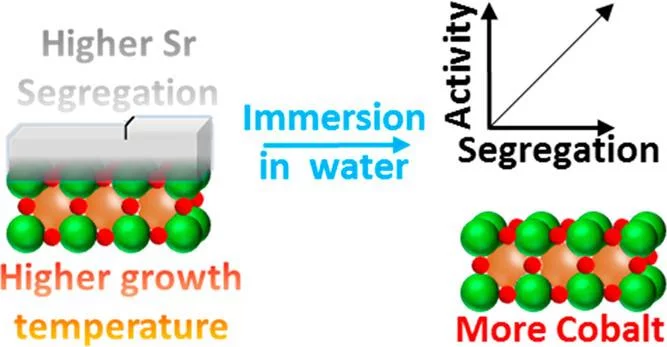
Surface Segregation Acts as Surface Engineering for the Oxygen Evolution Reaction on Perovskite Oxides in Alkaline Media
La1–xSrxCoO3-δ perovskites are potential catalysts for the anodic reaction of alkaline water electrolyzers, i.e., the oxygen evolution reaction (OER). It is well-known that La1–xSrxCoO3−δ perovskites can easily display strontium surface segregation, but how this influences the performance of La1–xSrxCoO3−δ perovskites as anodic electrode in alkaline water electrolyzers, particularly in terms of OER activity, has not been unveiled yet. This study focuses on La0.2Sr0.8CoO3−δ, which shows relatively high activity for the OER, and reveals the influence of the preparation temperature on the amount and morphology of segregated strontium-containing islands. Thin film samples were prepared at different temperatures by using pulsed laser deposition. Those samples were then characterized with synchrotron-based X-ray photoelectron spectroscopy “as prepared” and after being immersed in ultrapure water. We found that higher preparation temperatures enhance the segregation of strontium, which is then almost quantitatively removed by washing the samples with ultrapure water. After immersion in water, the samples expose a cobalt-rich surface. Investigating the OER activity as a function of the perovskite deposition temperature, it has been found that the higher the deposition temperature (i.e., the more extended the strontium segregation), the higher the OER activity. Such an effect has been linked to the higher amount of cobalt accessible after removing the strontium segregated islands.
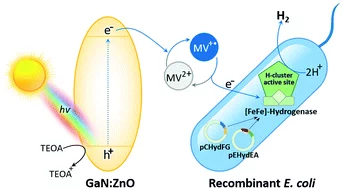
Photobiocatalytic H2 evolution of GaN:ZnO and [FeFe]-hydrogenase recombinant Escherichia coli
The need for sustainable, renewable and low-cost approaches is a driving force behind the development of solar-to-H2 conversion technologies. This study aims to develop a new strategy using a visible-light photocatalyst coupled to a biocatalyst for H2 production. Photocatalytic methyl viologen (MV2+) reduction activity was investigated to discover active oxynitrides. In comparative studies with LaTiO2N, BaTaO2N and Ta3N5, it was revealed that the suitable surface area, band gap and band edge potentials are some physical factors that are responsible for the photocatalytic behaviors of GaN:ZnO in MV2+ reduction. The activity is enhanced at higher concentrations and the alkaline pH of triethanolamine (TEOA). The expression of an active [FeFe]-hydrogenase from Escherichia coli (Hyd+E. coli) as a recombinant biocatalyst was confirmed by its MV˙+-dependent H2 production activity. In the photobiocatalytic system of GaN:ZnO and Hyd+E. coli, the rate of H2 production reached the maximum level in the presence of MV2+ as an electron mediator at neutral pH as a biocompatible condition. The present work reveals a novel hybrid system for H2 production using visible-light active GaN:ZnO coupled to Hyd+E. coli, which shows the feasibility of being developed for photobiocatalytic H2 evolution under solar light.
Examining the surface evolution of LaTiOxNy an oxynitride solar water splitting photocatalyst
LaTiOxNy oxynitride thin films are employed to study the surface modifications at the solid- liquid interface that occur during photoelectrocatalytic water splitting. Neutron reflectometry and grazing incidence x-ray absorption spectroscopy were utilised to distinguish between the surface and bulk signals, with a surface sensitivity of 3 nm.
Energy Conversion Processes with Perovskite-type Materials
Mixed oxides derived from the perovskite structure by combination of A- and B-site elements and by partial substitution of oxygen provide an immense playground of physico-chemical properties. Here, we give an account of our own research conducted at the Paul Scherrer Institute on perovskite-type oxides and oxynitrides used in electrochemical, photo(electro)chemical and catalytic processes aimed at facing energy relevant issues.
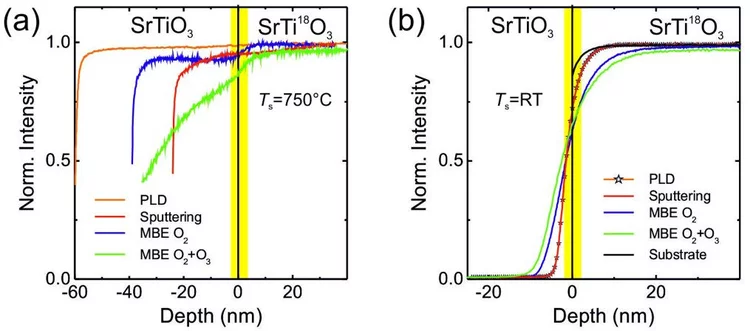
Oxygen diffusion in oxide thin films grown on SrTiO3
SrTiO3 thin films were grown on 18O-exchanged SrTiO3 single crystalline substrates by pulsed-laser deposition, rf sputtering, and oxide molecular-beam epitaxy to study their oxygen diffusion depth profiles using secondary ion mass spectrometry and elastic recoil detection analysis depth profiling. The oxygen depth profiling shows that SrTiO3 films prepared with the three different deposition techniques will take oxygen from the substrate, even at room temperature. This confirms that the substrate is one possible oxygen source for the growth of oxide thin films independent of the physical vapor deposition technique employed. It was also found that a reactive oxygen environment changes the oxygen composition of the substrate during the growth of a film and partly replaces 18O with 16O up to a depth of several tens of nm. These findings imply that SrTiO3 and therefore other ion conducting oxide substrates, which are commonly used as platforms for thin film growth, can be considered capricious in nature with respect to oxygen chemistry and lattice constants.
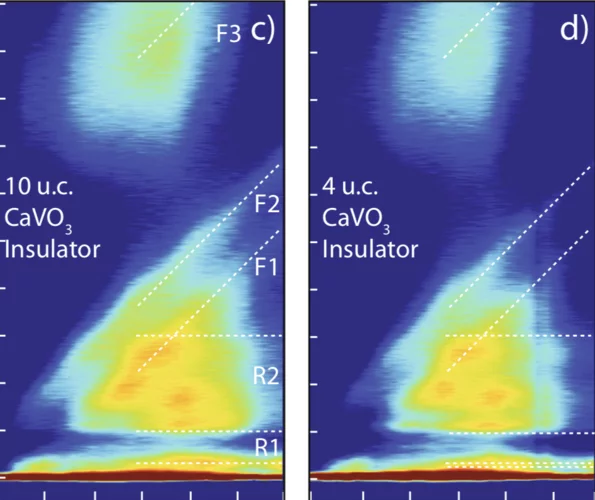
Electronic localization in CaVO3 films via bandwidth control
Understanding and controlling the electronic structure of thin layers of quantum materials is a crucial first step towards designing heterostructures where new phases and phenomena, including the metal-insulator transition (MIT), emerge. Here, we demonstrate control of the MIT via tuning electronic bandwidth and local site environment through selection of the number of atomic layers deposited.
Emergent magnetic monopole dynamics in macroscopically degenerate artificial spin ice
Magnetic monopoles, proposed as elementary particles that act as isolated magnetic south and north poles, have long attracted research interest as magnetic analogs to electric charge. In solid-state physics, a classical analog to these elusive particles has emerged as topological excitations within pyrochlore spin ice systems. We present the first real-time imaging of emergent magnetic monopole motion in a macroscopically degenerate artificial spin ice system consisting of thermally activated Ising-type nanomagnets lithographically arranged onto a pre-etched silicon substrate. factors are observed.
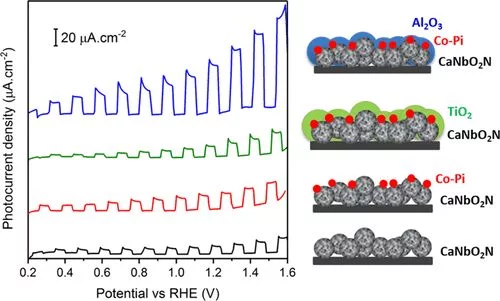
Improved Photoelectrochemical Water Splitting of CaNbO2N Photoanodes by CoPi Photodeposition and Surface Passivation
Photoelectrochemical (PEC) solar water splitting is a promising approach to convert solar energy into sustainable hydrogen fuel using semiconductor electrodes. Owing to their visible light absorption properties, oxynitrides have shown to be attractive photocatalysts for this application. In this study, the influence of the preparation method of CaNbO2N particles on their morphological and optical properties, and thereby their PEC performance, is investigated. The best performing CaNbO2N photoanode is produced by ammonolysis of Nb-enriched calcium niobium oxide.
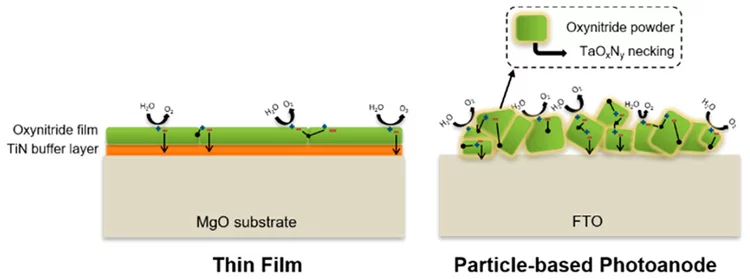
Oxynitride Thin Films versus Particle-Based Photoanodes: A Comparative Study for Photoelectrochemical Solar Water Splitting
The solar water splitting process assisted by semiconductor photocatalysts attracts growing research interests worldwide for the production of hydrogen as a clean and sustainable energy carrier. Because of their optical and electrical properties, several oxynitride materials show great promise for the fabrication of efficient photocatalysts for solar water splitting. This study reports a comparative investigation of particle- and thin-film-based photocatalysts using three different oxynitride materials.
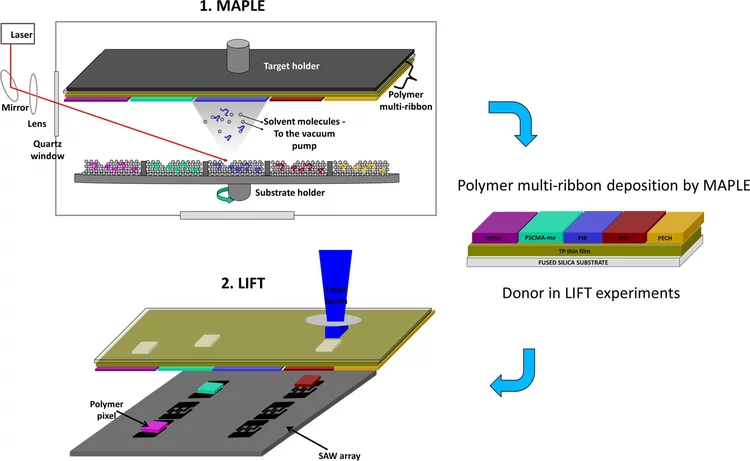
Highly selective surface acoustic wave e-nose implemented by laser direct writing
In this paper, we present an e-nose for the detection of volatile compounds based on an array of six surface acoustic wave (SAW) resonators coated with five different polymers (i.e. polyepichlorohydrin, polyisobutylene, polyethylenimine, (hydroxypropyl)methyl cellulose, and poly(styrene-co-maleic acid) partial isobutyl/methyl mixed ester, plus an uncoated SAW device used as reference.
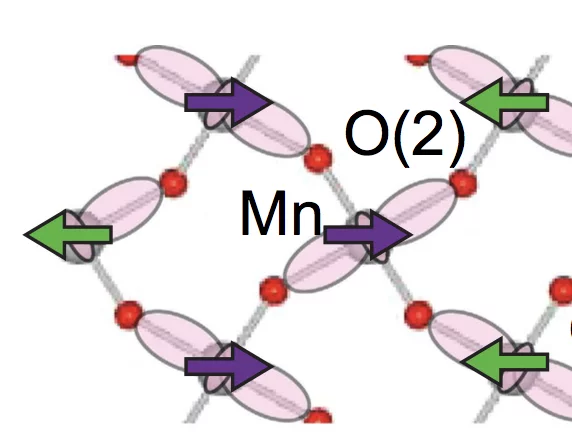
Relationship between crystal structure and multiferroic orders in orthorhombic perovskite manganites
We use resonant and nonresonant x-ray diffraction measurements in combination with first-principles electronic structure calculations and Monte Carlo simulations to study the relationship between crystal structure and multiferroic orders in the orthorhombic perovskite manganites, o−RMnO3 (R is a rare-earth cation or Y).
Rolling dopant and strain in Y-doped BiFeO3 epitaxial thin films for photoelectrochemical water splitting
We report significant photoelectrochemical activity of Y-doped BiFeO3 (Y-BFO) epitaxial thin films deposited on Nb:SrTiO3 substrates. The Y-BFO photoanodes exhibit a strong dependence of the photocurrent values on the thickness of the films, and implicitly on the induced epitaxial strain.
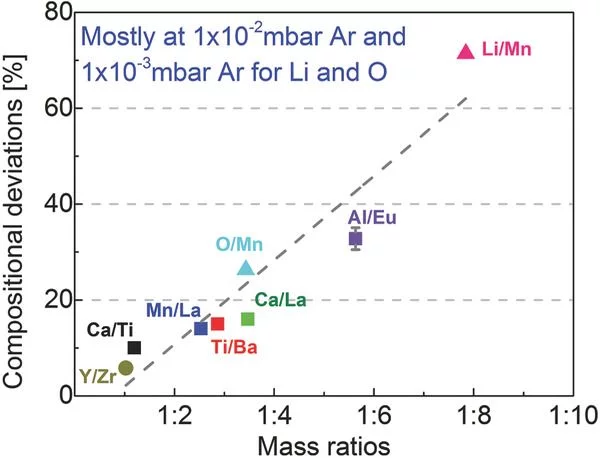
Influence of Plume Properties on Thin Film Composition in Pulsed Laser Deposition
Despite the apparent simplicity of pulsed laser deposition, consistent deposition of thin films with the desired thickness, composition, crystallinity, and quality still remains challenging. This article explores the influence of process parameters with respect to film thickness and composition, two key aspects for thin films which have a very strong effect on film properties, possible applications, and characterization.
Determination of Conduction and Valence Band Electronic Structure of LaTiOxNy Thin Films
The nitrogen substitution into the oxygen sites of several oxide materials leads to a reduction of the band gap to the visible-light energy range, which makes these oxynitride semiconductors potential photocatalysts for efficient solar water splitting. Oxynitrides typically show a different crystal structure compared to the pristine oxide material.
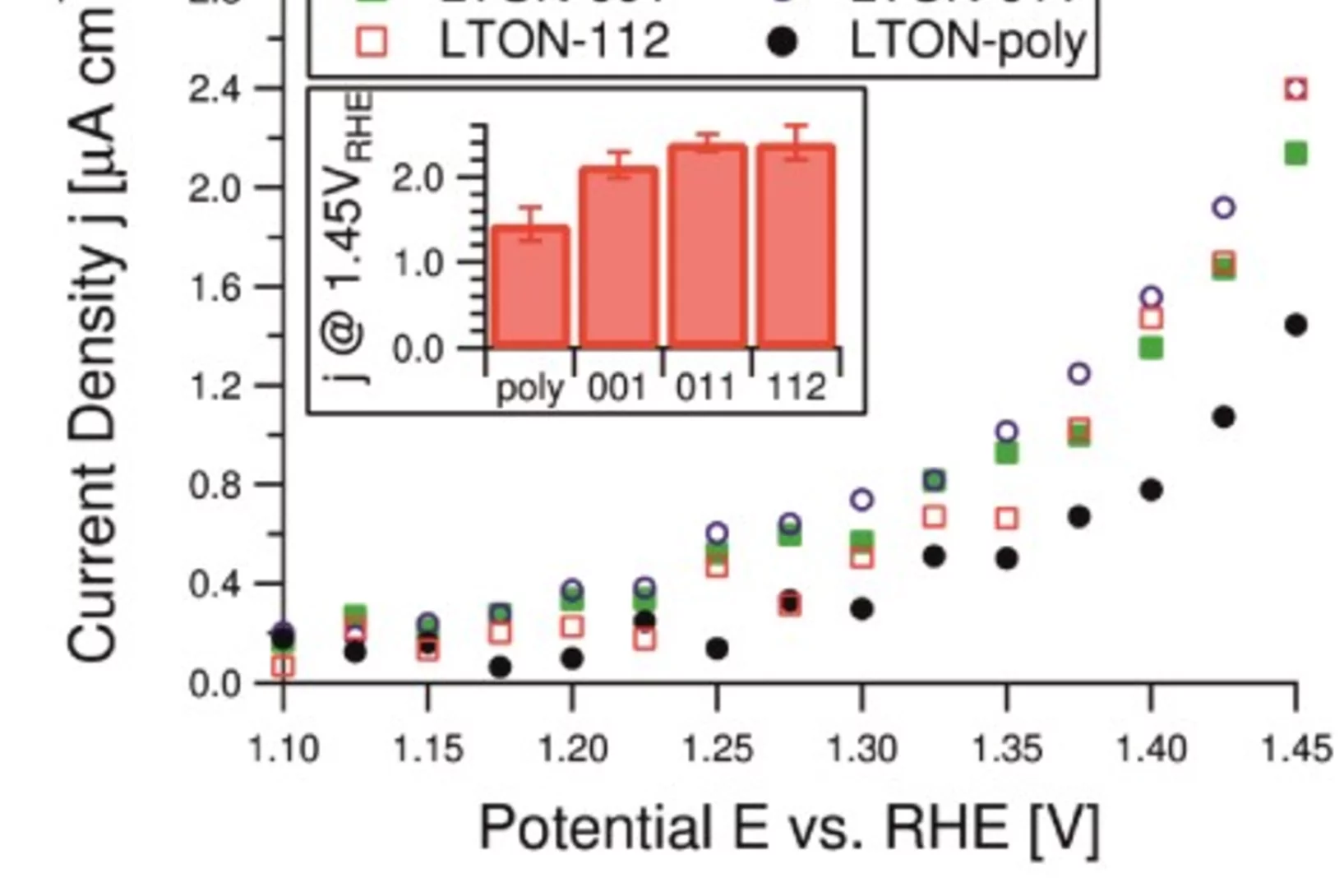
LaTiOxNy thin film model systems for photocatalytic water splitting: physicochemical evolution of the solid-liquid interface and the role of the crystallographic orientation
The size of the band gap and the energy position of the band edges make several oxynitride semiconductors promising candidates for efficient hydrogen and oxygen production under solar light illumination. The intense research efforts dedicated to oxynitride materials have unveiled the majority of their most important properties. However, two crucial aspects have received much less attention.
Tuning the multiferroic mechanisms of TbMnO3 by epitaxial strain
A current challenge in the field of magnetoelectric multiferroics is to identify systems that allow a controlled tuning of states displaying distinct magnetoelectric responses. Here we show that the multiferroic ground state of the archetypal multiferroic TbMnO3 is dramatically modified by epitaxial strain. Neutron diffraction reveals that in highly strained films the magnetic order changes from the bulk-like incommensurate bc-cycloidal structure to commensurate magnetic order.
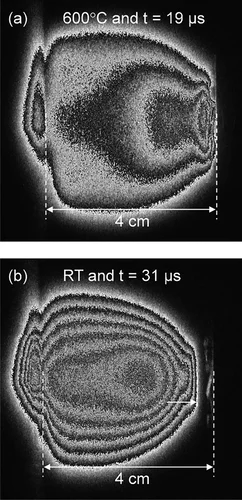
Pressure and temperature dependence of the laser-induced plasma plume dynamics
The influence of different background gases and substrate heating on the plasma plume dynamics from silver ablation is investigated by species selected time and space resolved imaging. The results provide a time-resolved understanding on how those process parameters affect the expansion: from a free expansion in vacuum with velocities exceeding 20'000 m/s to a very slow expansion in Ar at 1 × 10−1 mbar with arrival velocities of 280 m/s.
Structure and Conductivity of Epitaxial Thin Films of In-Doped BaZrO3‑Based Proton Conductors
Epitaxial thin films of the proton-conducting perovskite BaZr0.53In0.47O3−δH0.47−2δ, grown by pulsed laser deposition, were investigated in their hydrated and dehydrated conditions through a multitechnique approach with the aim to study the structure and proton concentration depth profile and their relationship to proton conductivity.