Thank You SLS
Our beamline scientists look back on 22 years of brilliant science made possible by the Swiss Light Source SLS.
Bright white coloring of Pacific cleaner shrimp revelead
In a study published in Nature Photonics, researchers at the Ben-Gurion University of the Negev, Israel, explain the bright, white-colored stripes from Pacific cleaner shrimps, one of the most efficient white reflectors found in nature.
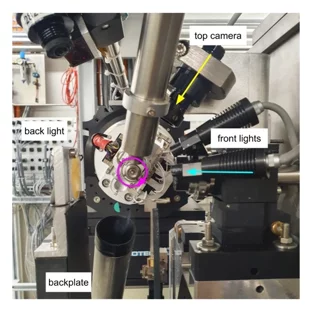
Progress of the X06DA-PXIII beamline upgrade: First light in the optics hutch
On June 7, 2023, the PXIII project team successfully shone the first light into the optics hutch at the upgraded X06DA-PXIII beamline. It is an essential first step for testing new hardware and software solutions that will be implemented at SLS2.0.
SDU: Software for high throughput automated data collection at SLS MX
The Smart Digital User (SDU) software for unattended data collection has been deployed at the macromolecular crystallography beamlines at the Swiss Light Source.
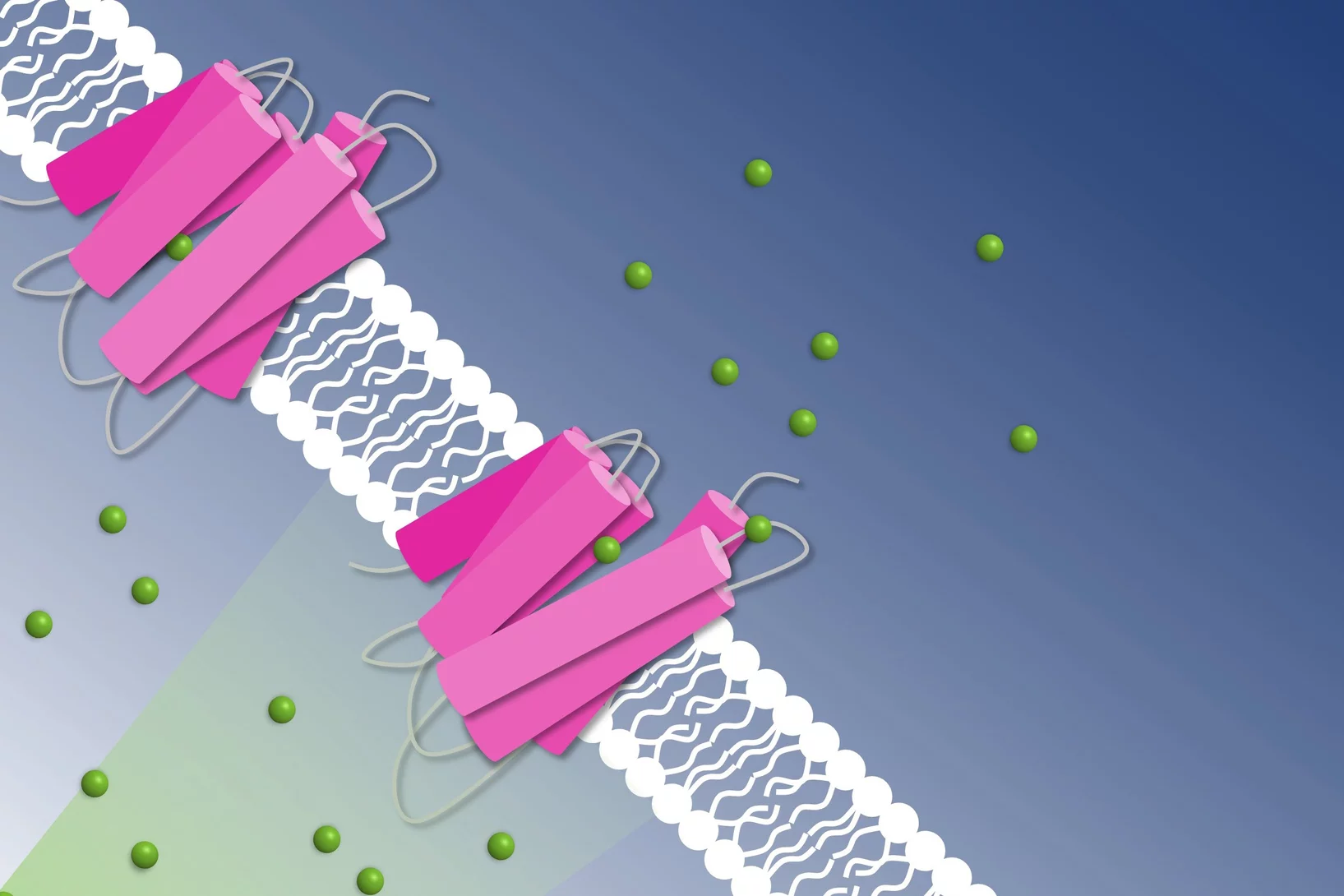
A new spin on sample delivery for membrane proteins
Proteins hover in front of the X-ray beam at a Swiss Light Source beamline. Now, spinning thin films bring on board these trickiest of proteins.
How to get chloride ions into the cell
A molecular movie shot at PSI reveals the mechanism of a light-driven chloride pump
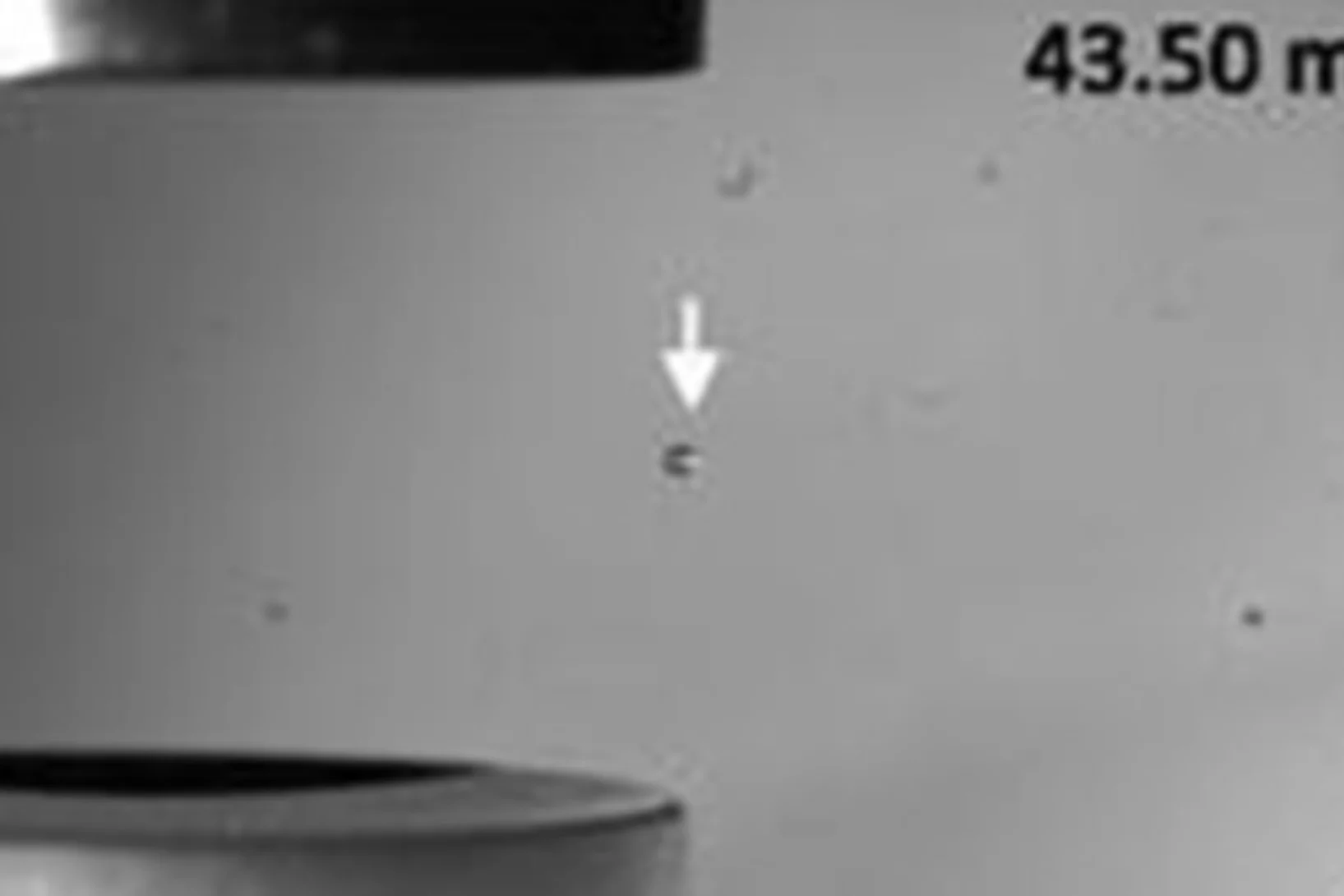
On-demand sample delivery article highlighted in "Applied Physics Letters"
An article on the on-demand sample delivery and protein crystallography using acoustic levitation has been selected in an Applied Physics Letters collection of papers on technology and application of acoustic tweezers.
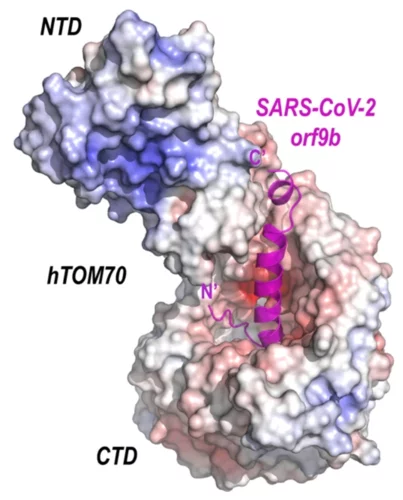
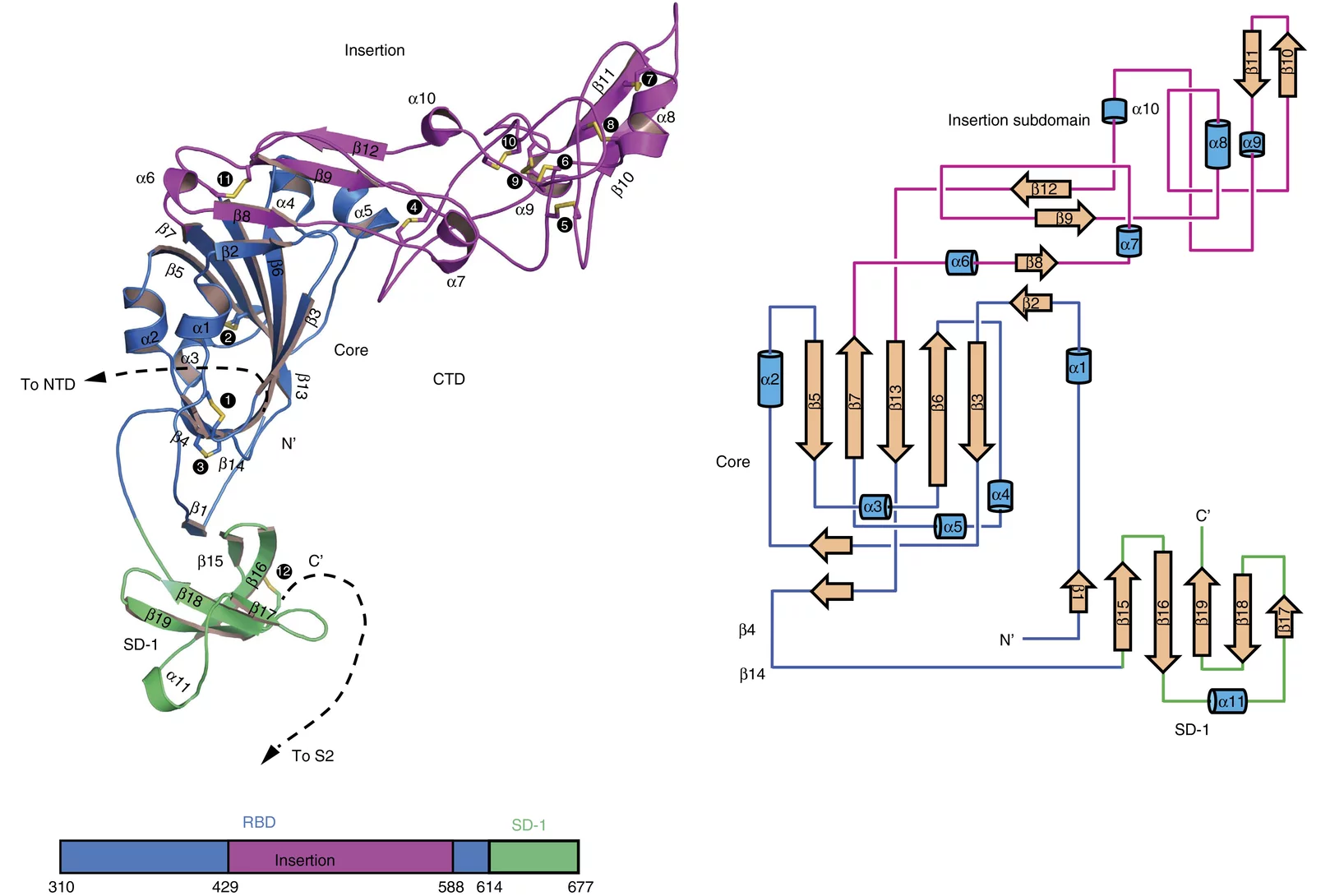
Crystal structure of SARS-CoV-2 Orf9b in complex with human TOM70 suggests unusual virus-host interactions
In a study published in Nature Communications, researchers at the NHC Key Laboratory of Systems Biology of Pathogens in Beijing, China, in collaboration with the Paul Scherrer Institut characterize the interactions of SARS-CoV-2 orf9b and human TOM70 biochemically, and they determine the 2.2 Å crystal structure of the TOM70 cytosolic domain with a bound SARS-CoV-2 orf9b peptide.
Mode d’action du remdesivir contre le coronavirus
En coopération avec le PSI, des chercheurs de l’Université Goethe de Francfort ont probablement découvert un nouveau mécanisme d’action inconnu du remdesivir.
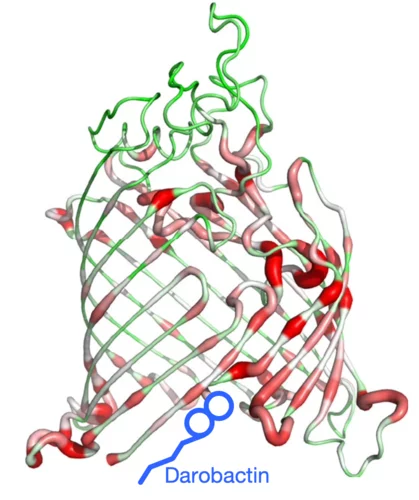
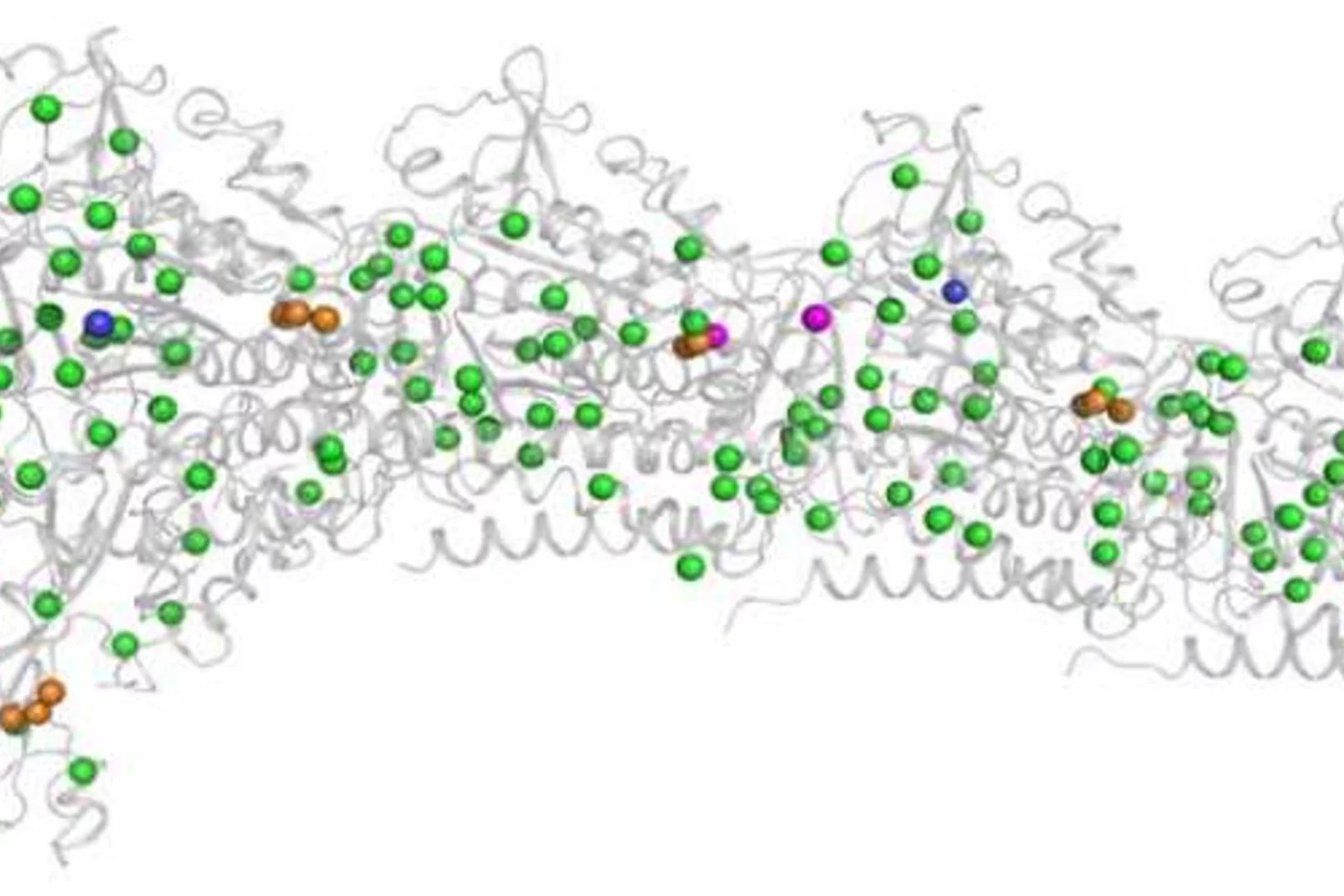
Combating antimicrobial resistance
In a study addressing the global health threat of drug resistance, researchers at the Biozentrum, University of Basel, have revealed how a new antibiotic, Darobactin, binds to the external membrane of gram-negative bacteria.
Recherche sur le Covid-19: stratégie antivirale à double effet
Des scientifiques de Francfort ont identifié un éventuel point faible du virus SARS-CoV-2. Ils ont effectué une partie de leurs mesures à la Source de Lumière Suisse SLS du PSI. Leurs résultats de recherche paraissent cette semaine dans la revue spécialisée Nature.
First MX results of the priority COVID-19 call
The Dikic group at the Goethe University in Frankfurt am Main, Germany has published the first results following the opening of the "PRIORITY COVID-19 Call” at SLS.
SLS MX beamtime update
Update of the SLS MX beamline operation during the COVID-19 period
A crystal-clear picture
Fast and accurate data collection for macromolecular crystallography using the JUNGFRAU detector.
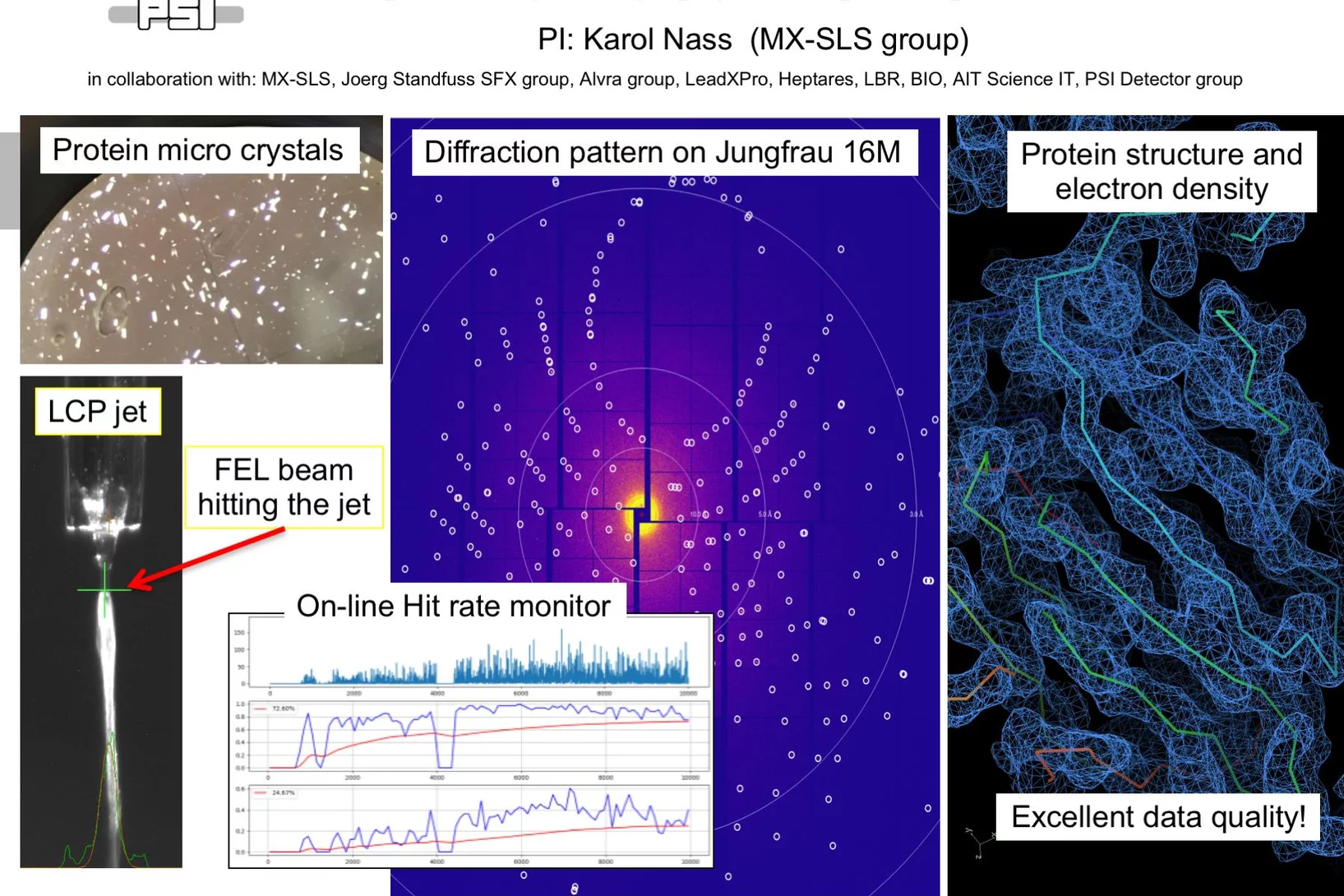
First serial femtosecond crystallography (SFX) pilot user experiment at SwissFEL
On the 7th to 12th of August 2018, a collaborative group of scientists from the Paul Scherrer Institute and members of the LeadXpro and Heptares pharmaceutical companies led by Karol Nass (PSI macromolecular crystallography MX-SLS group) performed the first serial femtosecond crystallography (SFX) pilot user experiment at the SwissFEL X-ray free electron laser (XFEL).
Rhine-Knee Regiomeeting 2018
Since it was first established in 1987, the annual Regio-Meeting has been instrumental in facilitating interactions in the structural biology community in southwestern Germany, the eastern region of France and an expanding area of Switzerland. It is set as an informal event to foster young scientists to discuss their research results in an international context. The 2018 edition will take place in the heart of Switzerland in Emmetten from September 26 to 28, 2018. Registration and abstract submission deadline: September 7, 2018.
HERCULES at the Swiss Light Source
In the week of March 18-23 PSI welcomes 20 PhD students and postdocs taking part in the HERCULES 2018 school on Neutron and Synchrotron Radiation. They will attend lectures and perform two days of practical courses at several beam lines of the Swiss Light Source.
In Situ Serial Crystallography 2 Workshop at the SLS
A workshop dedicated to the presentation of the in meso in situ serial crystallography (IMISX) method (Huang et al. 2015, 2016 ActaD) for the X-ray structure determination of membrane proteins is organised at the Swiss Light Source at PSI for the second time. It will be held between November 27 and 29, 2017.
Towards understanding of human betacoronavirus HKU1 life cycle
Researchers from China and USA join forces with Swiss Light Source (SLS) macromolecular crystallography (MX) beamline scientists in a study, which aims at understanding an important step in the life cycle of the human betacoronavirus HKU1.
1000 Structures solved at X06DA-PXIII
The macromolecular crystallography beamline X06DA-PXIII has reached 1,000 structures in the Protein Data Bank (PDB) on February 22, 2017.
First protein structure solved using the JUNGFRAU detector!
JUNGFRAU is a charge-integrating, two-dimensional pixel detector developed at the Paul Scherrer Institut for use at free-electron lasers, in particular SwissFEL, and synchrotron light sources. On the 10th October, the first protein crystallography experiment using the JUNGFRAU detector, was performed at the beamline X06SA (PXI) of the Swiss Light Source by the members of the Protein Crystallography and Detectors groups at PSI.
Call for expressions of interest: Beamline partners at the SLS for PX II and PX III
We invite companies and institutions to secure access to the beamlines X10SA/PX II and X06DA/PX III through a long term contract.
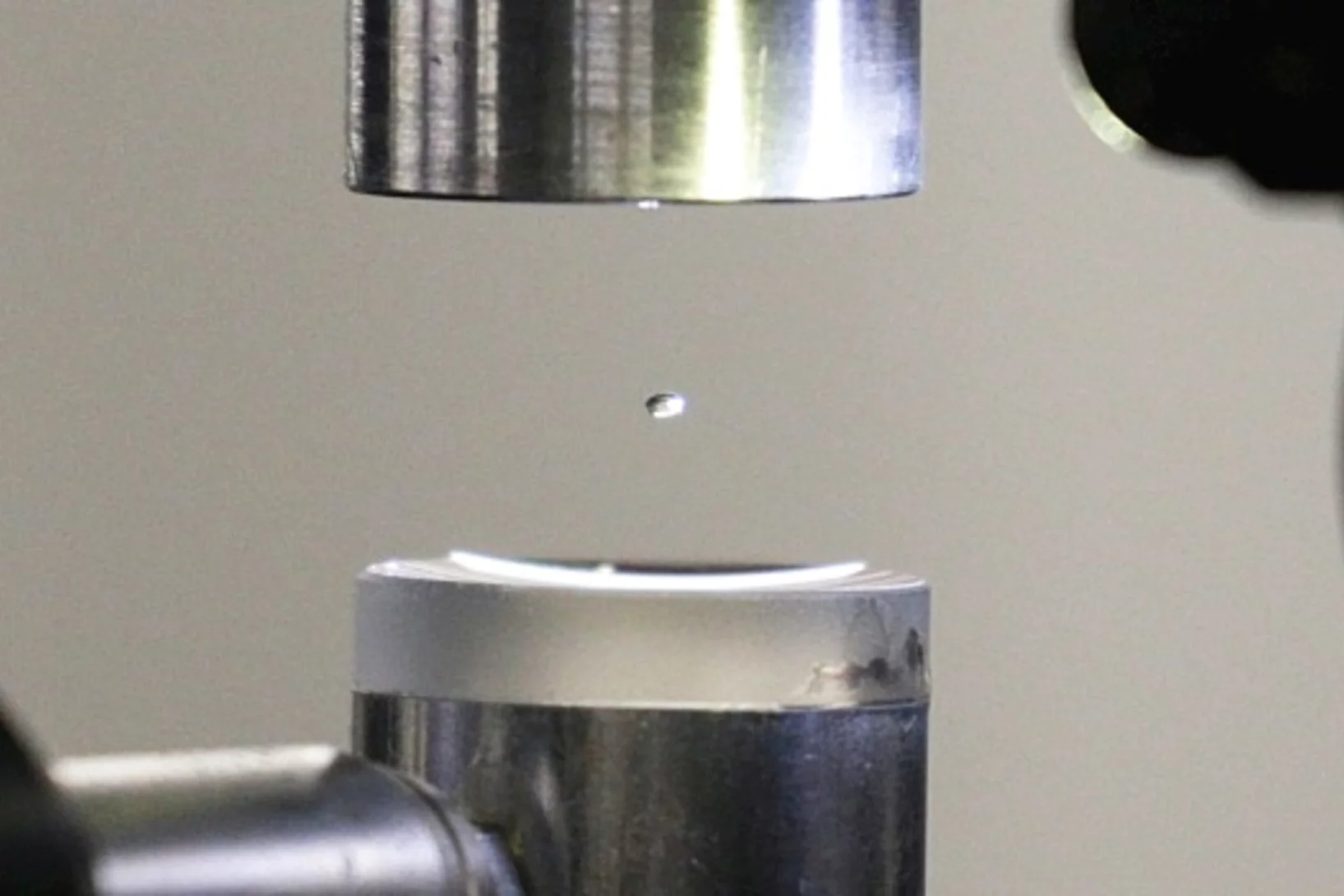
Expérience dans une goutte en lévitation
La structure exacte des protéines est normalement déterminée au PSI par la technique de diffraction des rayons X. Deux scientifiques du PSI viennent de l’améliorer de façon astucieuse: au lieu d’immobiliser les protéines, ils les ont étudiées dans une goutte de liquide en lévitation.
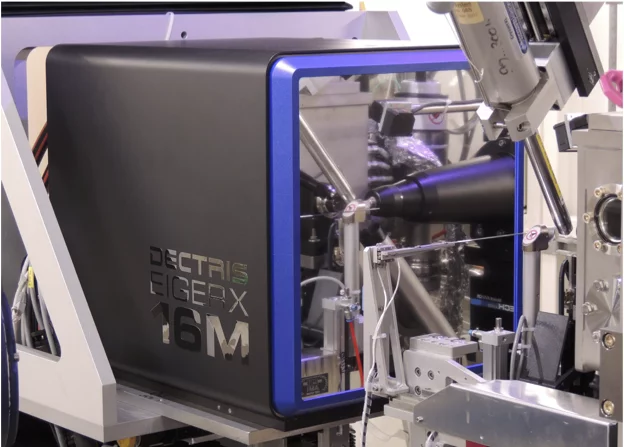
First EIGER X 16M in operation at the Swiss Light Source
The macromolecular crystallography beamline X06SA at the Swiss Light Source, a synchrotron operated by Paul Scherrer Institute, is the first one in the world to upgrade its detector to an EIGER X 16M.
In Situ Serial Crystallography Workshop at the SLS
The Macromolecular Crystallography group at SLS is organizing a three days workshop on in situ serial crystallography (http://indico.psi.ch/event/issx) between November 17 and 19, 2015. It will be dedicated in the presentation of a novel method facilitating the structure determination of membrane proteins, which are highly important pharmaceutical targets but are difficult to handle using 'classical' crystallographic tools. Designed for 20 Ph.D. students, postdocs and young scientists from both academia and industry, the workshop will consist of introductory lectures, followed by hands-on practicals on in meso or lipidic cubic phase (LCP) crystallization, on in situ serial crystallography data collection using a micro-sized beam and on data processing.
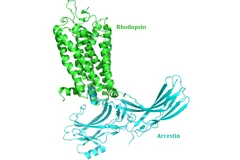
New insight into receptor signalling
A team of 72 investigators across 25 institutions including researchers from the Paul Scherrer Institut obtained the X-ray structure of a rhodopsinàarrestin complex, which represents a major milestone in the area of G-protein-coupled-receptor (GPCR), a protein family recognized in the award of the 2012 Nobel Prize in Chemistry.
L’union fait la force
Décrypter les molécules au SwissFEL et à la SLSLes protéines sont un objet de recherche convoité, mais récalcitrant. Leur étude est aujourd’hui facilitée par une nouvelle méthode développée à l’aide d’un laser à rayons X à électrons libres comme le futur SwissFEL du PSI. Elle consiste à exposer à intervalles rapprochés de petits échantillons identiques de protéines à de la lumière de type rayons X. On contourne ainsi un problème majeur auquel la recherche sur les protéines s’est heurtée jusqu’ici: produire des échantillons de taille suffisante.
Obtenir plus rapidement le portrait d’une protéine
Tous les êtres vivants, de la bactérie à l’être humain, ont besoin de protéines pour l’accomplissement de leurs fonctions vitales. La manière dont les protéines remplissent leurs tâches dépend de leur structure. Des chercheurs de l’Institut Paul Scherrer (PSI) ont développé une méthode novatrice, qui permet de déterminer plus rapidement la structure cristalline des protéines, grâce à de la lumière de type rayons X. Elle pourrait ainsi accélérer le développement de nouveaux médicaments. Leur étude sera publiée le 15 décembre dans la revue spécialisée « Nature Methods ».
Wie der Körper Fremdes von Eigenem unterscheiden - wesentliche Struktur aufgeklärt
Ähnlich einem Schredder zerlegt das Immunoproteasom Eiweisse in kleine Bruchstücke, die dann an der Oberfläche der Zelle präsentiert werden. Werden diese Eiweissteile als körperfremd erkannt, vernichtet das Immunsystem die Zelle. Bei Autoimmunerkrankungen wie Rheuma ist dieser Prozess gestört. Helfen könnte da, das Immunoproteasom zu hemmen. Mit Hilfe von Messungen am Paul Scherrer Institut ist es nun erstmals gelungen, die Struktur des Immunoproteasoms aufzuklären und Angriffsstellen für neue Medikamente aufzuzeigen.Cette actualité n'existe qu'en anglais et allemand.
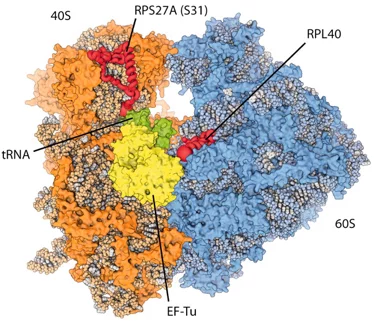
New insights into the cell’s protein factory
Eukaryotic ribosomes are among the most complex cellular machineries of the cell. These large macromolecular assemblies are responsible for the production of all proteins and are thus of pivotal importance to all forms of life. Two independent research groups at the ETH Zürich and the Institute of Genetics and Molecular and Cellular Biology in Strasbourg have obtained new insights into the atomic structure of the eukaryotic ribosome. The results have been published in the journal Science.