For the first time, a molecular movie has captured in detail the process of an anion transported across the cell membrane by a light-fueled protein pump. Publishing in Science, the researchers utilized the unique synergy of a Free Electron Laser (SwissFEL) and synchrotron light source (SLS) offered by PSI to unravel the mystery of how light energy initiates the pumping process − and how nature made sure there is no anion leakage back outside.
Many bacteria and unicellular algae have light-driven pumps in their cell membranes: proteins that change shape when exposed to photons such that they can transport charged atoms in or out of the cell. Thanks to these pumps, their unicellular owners can adjust to the environment’s pH value or salinity.
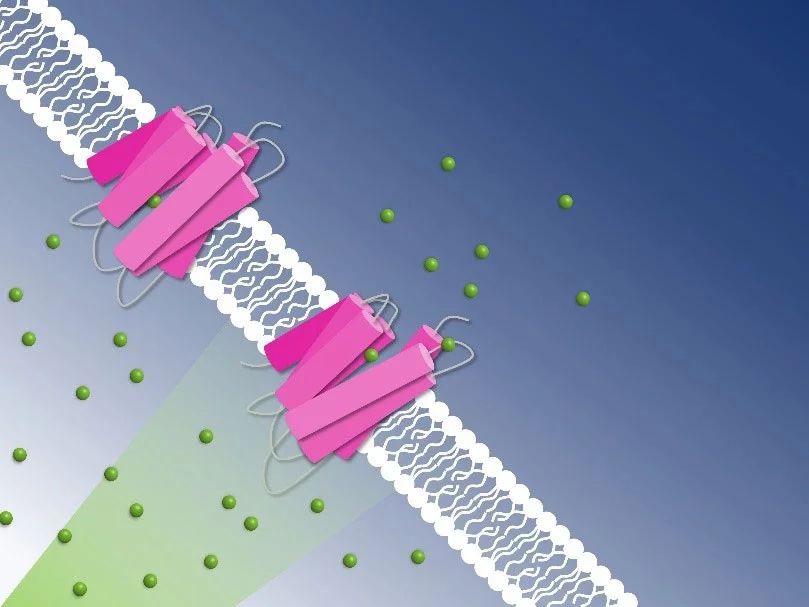
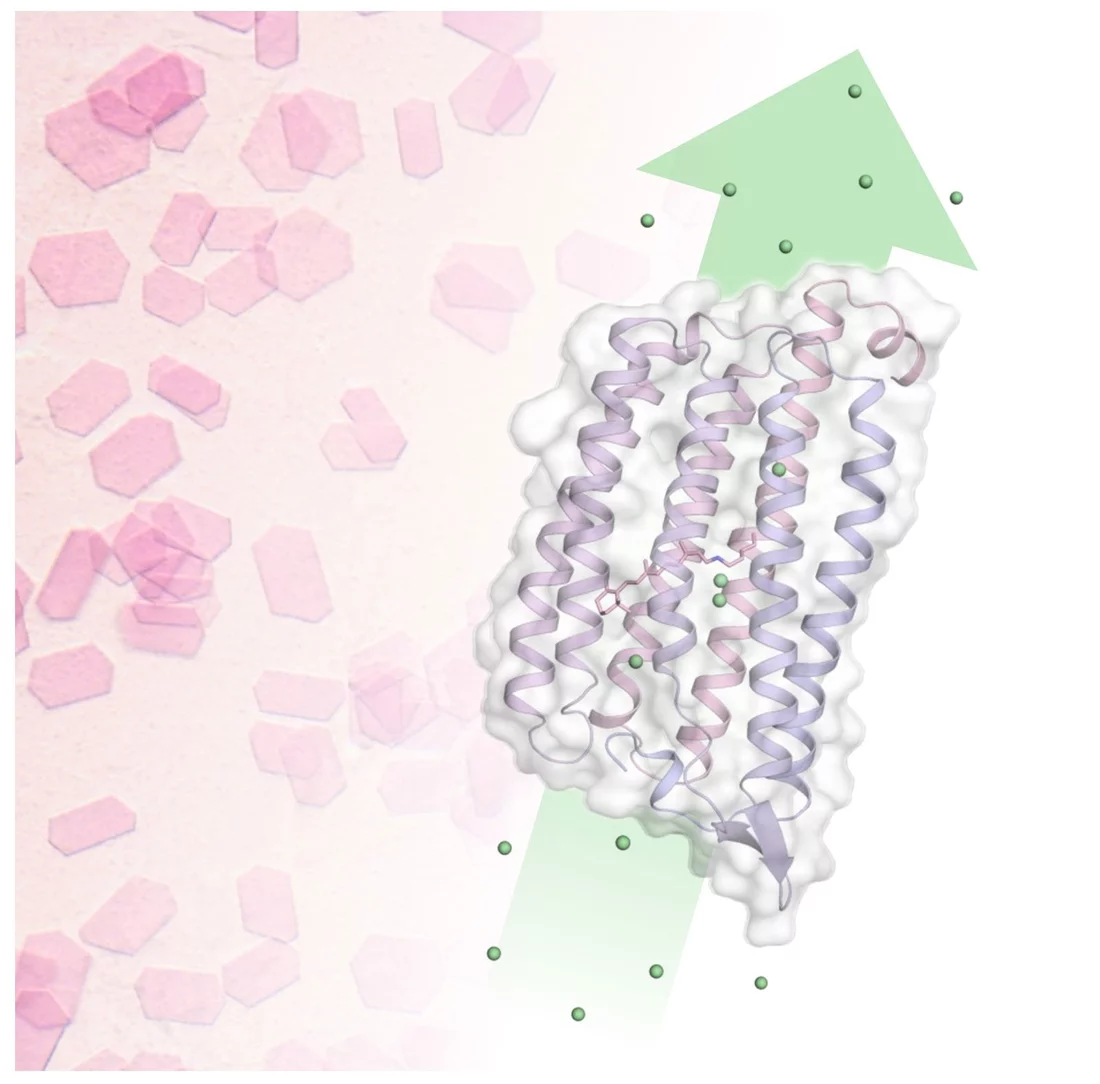
One such bacteria is Nonlabens marinus, first discovered in 2012 in the Pacific Ocean. Among others, it possesses a rhodopsin protein in its cell membrane which transports chloride anions from outside the cell to its inside. Just like in the human eye, a retinal molecule bound to the protein isomerizes when exposed to light. This isomerization starts the pumping process. Researchers now gained detailed insight into how the chloride pump in Nonlabens marinus works.
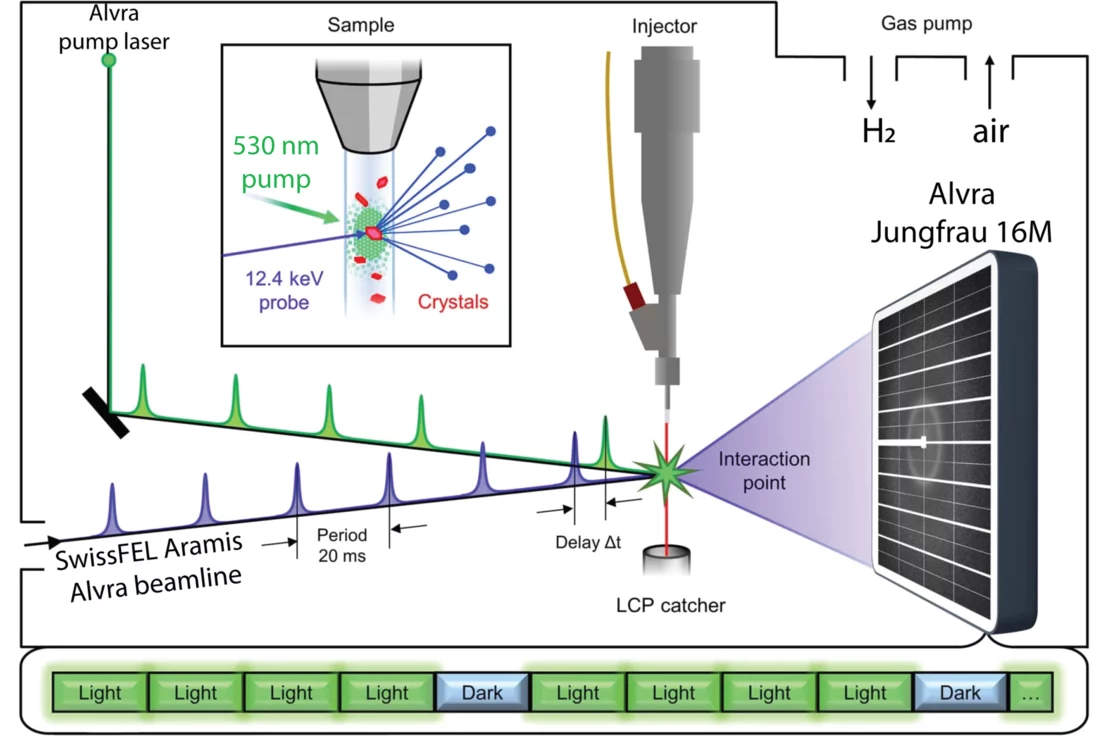
The study was led by Przemyslaw Nogly, once a postdoc at PSI and now an Ambizione Fellow and Group Leader at ETH Zürich, in close collaboration with the ALVRA team at SwissFEL and the MX team at the SLS. It is one of the first studies to fully combine experimental capabilities at these large-scale research facilities, bridging the gap in time resolution to record a full molecular movie of a protein at work. Slower dynamics in the millisecond-range were investigated via time-resolved serial crystallography at SLS while faster, up to picosecond, events were captured at SwissFEL - then both sets of data were put together.
With the advent of FELs, the possibility of recording biological molecules in action became a reality, allowing observation of reactions from femto- to microseconds. However, for the slower steps in the millisecond to second range, FELs are not suitable. Expanding the experimental techniques to the synchrotron closes this gap. “At PSI, we are in the unique position to offer both setups to users in a synergetic way”, Florian Dworkowski, beamline scientist at the SLS, explains. “With the advent of the 4th generation upgrade for the SLS, we plan to expand the time resolution at the synchrotron even to the microsecond regime, closing the gap completely”.
“In one paper, we exploit the advantages of two state-of-the-art facilities to tell the full story of this chloride pump,” Nogly says. Jörg Standfuss, co-author of the study who built up a PSI team dedicated to creating such molecular movies, adds: “This combination enables first-class biological research as would only be possible at very few other places in the world beside PSI.”
No backflow
As the study has revealed, the chloride anion is attracted by a positively charged patch of the rhodopsin protein in Nonlabens marinus’ cell membrane. Here, the anion enters the protein and finally binds to a positive charge at the retinal molecule inside. When retinal isomerizes due to light exposure and flips over, it drags the chloride anion along and thus transports it a bit further inside the protein. “This is how light energy is directly converted into kinetic energy, triggering the very first step of the ion transport,” Sandra Mous says, a PhD student in Nogly’s group and first author of the paper.
Being on the other side of the retinal molecule now, the chloride ion has reached a point of no return. From here, it goes only further inside the cell. An amino acid helix also relaxes when chloride moves along, additionally obstructing the passage back outside. “During the transport, two molecular gates thus make sure that chloride only moves in one direction: inside,” Nogly says. One pumping process in total takes about 100 milliseconds.
Two years ago, Jörg Standfuss, Przemyslaw Nogly and their team unravelled the mechanism of another light-driven bacterial pump: the sodium pump of Krokinobacter eikastus. Researchers are eager to discover the details of light-driven pumps because these proteins are valuable optogenetic tools: genetically engineered into mammalian neurons, they make it possible to control the neurons activities by light and thus research their function.
Text: Brigitte Osterath
Contact
Dr. Przemyslaw Nogly
Head of the Protein Structural Dynamics Group
Institute of Molecular Biology and Biophysics
ETH Zürich, Hönggerbergring 64, 8093 Zürich, Switzerland
Telephone: +41 44 633 07 19, e-mail: przemyslaw.nogly@mol.biol.ethz.ch
Dr. Jörg Standfuss
Head of the Time-resolved Crystallography Group
Laboratory of Biomolecular Research
Paul Scherrer Institute, Forschungsstrasse 111, 5232 Villigen PSI, Switzerland
Telephone: +41 56 310 25 86, e-mail: joerg.standfuss@psi.ch
Dr. Florian Dworkowski
Beamline Scientist
Paul Scherrer Institute, Forschungsstrasse 111, 5232 Villigen PSI, Switzerland
Telephone: +41 56 310 35 84, e-mail: florian.dworkowski@psi.ch
Dr. Claudio Cirelli
Beamline scientist at Alvra
Paul Scherrer Institute, Forschungsstrasse 111, 5232 Villigen PSI, Switzerland
Telephone: +41 56 310 36 82, e-mail: claudio.cirelli@psi.ch
Original Publication
Dynamics and mechanism of a light-driven chloride pump
Sandra Mous, Guillaume Gotthard, David Ehrenberg, Saumik Sen, Tobias Weinert, Philip J. M. Johnson, Daniel James, Karol Nass, Antonia Furrer, Demet Kekilli, Pikyee Ma, Steffen Brünle, Cecilia Maria Casadei, Isabelle Martiel, Florian Dworkowski, Dardan Gashi, Petr Skopintsev, Maximilian Wranik, Gregor Knopp, Ezequiel Panepucci, Valerie Panneels, Claudio Cirelli, Dmitry Ozerov, Gebhard Schertler, Meitian Wang, Chris Milne, Joerg Standfuss, Igor Schapiro, Joachim Heberle, Przemyslaw Nogly
Science, 03 February 2022 (online)
Additional Information
Copyright
PSI provides image and/or video material free of charge for media coverage of the content of the above text. Use of this material for other purposes is not permitted. This also includes the transfer of the image and video material into databases as well as sale by third parties.