In Situ Serial Crystallography Workshop at the SLS
The Macromolecular Crystallography group at SLS is organizing a three days workshop on in situ serial crystallography (http://indico.psi.ch/event/issx) between November 17 and 19, 2015. It will be dedicated in the presentation of a novel method facilitating the structure determination of membrane proteins, which are highly important pharmaceutical targets but are difficult to handle using 'classical' crystallographic tools. Designed for 20 Ph.D. students, postdocs and young scientists from both academia and industry, the workshop will consist of introductory lectures, followed by hands-on practicals on in meso or lipidic cubic phase (LCP) crystallization, on in situ serial crystallography data collection using a micro-sized beam and on data processing.
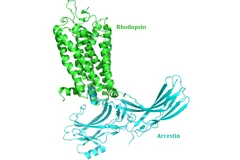
New insight into receptor signalling
A team of 72 investigators across 25 institutions including researchers from the Paul Scherrer Institut obtained the X-ray structure of a rhodopsinàarrestin complex, which represents a major milestone in the area of G-protein-coupled-receptor (GPCR), a protein family recognized in the award of the 2012 Nobel Prize in Chemistry.
Together, not alone
Decoding biomolecules at SwissFEL and SLSProteins are a coveted but stubborn research object. A method developed for x-ray free-electron lasers and PSI’s future SwissFEL should now help researchers to make good headway in this field. It involves x-raying many small, identical protein samples consecutively at short intervals, thereby avoiding the main problem that protein research has faced thus far: producing samples in a sufficient size.
Shortcut to protein portraits
All living organisms, from bacteria to humans, rely on proteins to perform their vital functions. How these proteins accomplish their tasks depends on their structure. Researchers from the Paul Scherrer Institute have now devised a novel method to determine the crystal structure of proteins using X-ray light, which could also hasten the development of new drugs in future. The study will be published in the journal Nature Methods on 15 December.
How the body distinguishes between self and non-self – important structures explained
Like a shredder, the immunoproteasome cuts down proteins into peptides that are subsequently presented on the cellular surface. The immune system can distinguish between self and non-self peptides and selectively kills cells that present non-self peptides at their surface. In autoimmune diseases, this mechanism is deregulated. However, inhibition of the immunoproteasome may alleviate disease symptoms and progression. With the help of measurements taken at the Paul Scherer Institute, scientists have now succeeded in determining the first structure of an immunoproteasome.
New insights into the cell’s protein factory
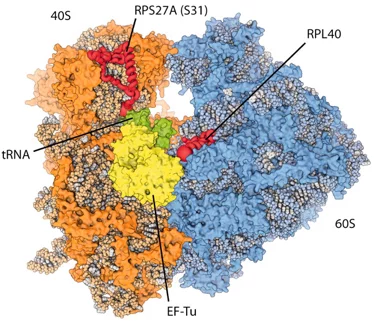
Eukaryotic ribosomes are among the most complex cellular machineries of the cell. These large macromolecular assemblies are responsible for the production of all proteins and are thus of pivotal importance to all forms of life. Two independent research groups at the ETH Zürich and the Institute of Genetics and Molecular and Cellular Biology in Strasbourg have obtained new insights into the atomic structure of the eukaryotic ribosome. The results have been published in the journal Science.
Dem Rätsel der Centriolen-Bildung auf der Spur
In menschlichen Zellen finden sich stammesgeschichtlich sehr alte Funktionseinheiten, die als Centriolen bezeichnet werden. Ein Forscherteam vom PSI und der ETH Lausanne hat nun erstmals ein Modell für die Bildung der Centriolen vorgestellt. Das erstaunende Ergebnis ist, dass die Neuner-Symmetrie des Centriols durch die Fähigkeit eines einzelnen Proteins sich selbst zu organisieren zustande kommt.This news release is only available in French and German.