Decoding biomolecules at SwissFEL and SLS
Proteins are a coveted but stubborn research object. A method developed for x-ray free-electron lasers and PSI’s future SwissFEL should now help researchers to make good headway in this field. It involves x-raying many small, identical protein samples consecutively at short intervals, thereby avoiding the main problem that protein research has faced thus far: producing samples in a sufficient size.
Without proteins, our bodies wouldn't even work. These multifaceted transformation artists sit in every cell and control our bodily functions. One of the many examples is the so-called chemokine receptors in our immune system. They ensure that our defence cells move to wherever they can combat unwelcome intruders such as viruses or bacteria the most effectively.
Thanks to their key functions, proteins are especially a coveted research object for drug development. Researching them, however, is hard work. One of the key methods in biological research involves crystallising biomolecules, x-raying them and calculating the structure of the molecules from the light deflected as a result. This method has also been used at PSI's Swiss Light Source for many years to gain profound insights into the structure of biomolecules.
Only thanks to crystallisation can the molecules be displayed. However, the fragile and considerably more complex proteins don't make life as easy for the researchers as sea salt crystals, which remain behind all by themselves when seawater is evaporated: "In the case of some proteins, an efficient crystallisation takes several years; for others, such as the chemokine receptors, we are unable to achieve the crystal size necessary for the experiments," says Jörg Standfuss, who specialises in researching these pig-headed contemporaries.
Unlocking the molecular structure bit by bit
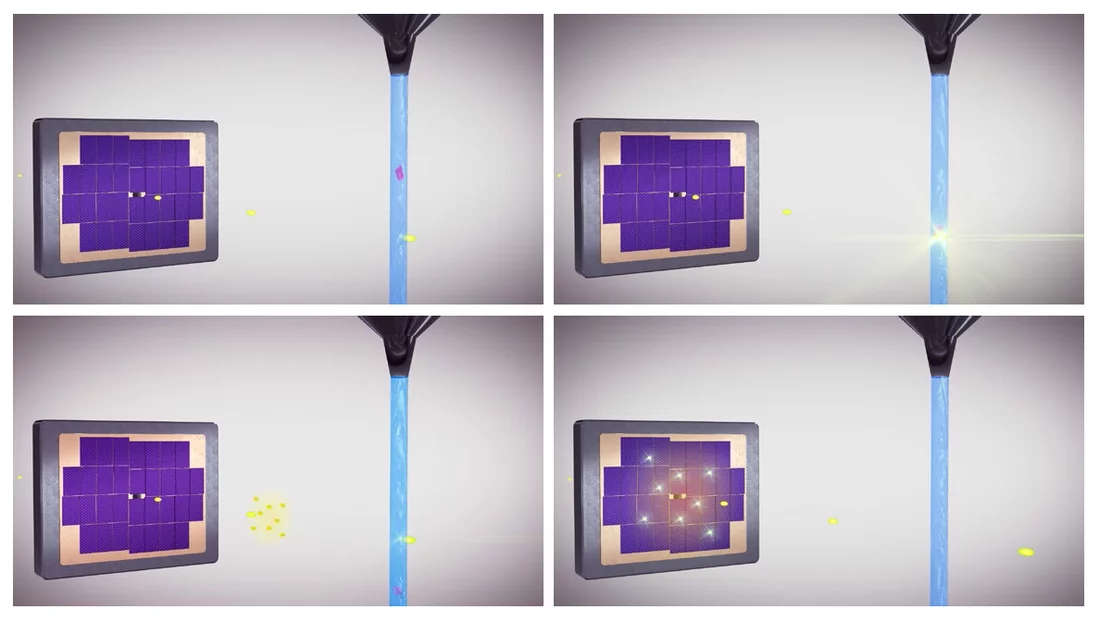
Currently, the most promising solution to this plight is known as serial crystallography. Instead of rotating individual large crystals in order to x-ray and depict them from as many different angles as possible, as has been the case so far, thousands of small, identical crystals are injected into the x-ray beam in succession (hence the term "serial"). The light beams deflected when the beam and the individual crystals collide are recorded and these recordings merged. With a sufficiently large number of crystals, a precise image of the protein's structure emerges.
In serial crystallography, crystals are injected into an x-ray beam. When the beam and crystal collide, light beams are deflected. The deflected light beams are recorded by a detector. The structure of the crystals can be determined from the light patterns that many identical crystals create on the detector.
The method was developed for use on x-ray free-electron lasers like SwissFEL, which is currently under construction at PSI. The high radiation intensity of the x-ray lasers destroys every crystal within an extremely short time, ruling out the possibility of a rotation experiment. This doesn't do the measurement result any harm; quite the contrary, in fact: the beam bombardment's pulses are so short that the deflected light beams of the completely undamaged crystals can be recorded. Moreover, the method works at room temperature, which means that the molecules don't have to be cooled to below minus 173 degrees Celsius, as is usually the case.
Serial crystallography will enable us to study proteins in their natural form,
beams Standfuss. Taking it one step further, the biologist hopes to finally see proteins that change their form depending on their function in action: We can achieve this by simultaneously inducing a reaction in a series of identical crystals and staggering the injection of the crystals into the x-ray beam.
This gives the researchers an image of the structure at a particular point during the reaction, which they can piece together to make a film.
Serial crystallography will be available at SwissFEL as soon as the facility enters into operation. The x-ray free-electron laser will fire 100 x-ray pulses per second. One of the challenges is to inject the crystals in such a way that they meet the pulses with pinpoint accuracy,
says Christopher Milne, who is developing the SwissFEL experimentation station that will offer serial crystallography. Although smaller crystals are usually easier to produce, their possible number is limited for many proteins. No crystal should be injected into the void and lost for the experiment.
One eye on synergy
The research and development work is well underway. It is not just limited to SwissFEL, either. The methods developed for the sample injection on x-ray free-electron lasers might well expand the possibilities for studying proteins on synchrotron light sources, too,
stresses Standfuss. That's important because the number of experimentation stations on x-ray free-electron lasers will be heavily limited even with SwissFEL operational, which means you will be restricted to experiments there that are absolutely impossible on other facilities.
Initial studies reveal the great potential of serial crystallography on synchrotron light sources,
says Meitian Wang, who is in charge of the beamline at the SLS, where such experiments are to take place in future. The priority now at PSI is to hone the method based on concrete examples. These include one of the chemokine receptors mentioned at the outset - a protein, which plays a key role in combatting inflammatory processes in the body. And it is one of those proteins that could not be crystallised successfully in the past.
The field is in a state of flux and developing extremely rapidly. However, Milne is convinced that things will calm down somewhat once SwissFEL is up and running: Your average biologist simply wants to measure, not keep grappling with a new method. They just want the best possible result and it's our job to make sure they get it.
Text: Paul Scherrer Institut/Martina Gröschl