Researchers elucidate how botulinum neurotoxin A binds to its protein receptor and thus provide a basis for the development of new drugs
Botulinum neurotoxin A, better known as botox, is a highly dangerous toxin that causes paralysis in man that may prove fatal. In cosmetic applications the paralysing action of small doses is used in a specific manner for the temporary elimination of wrinkles and in medicine as a treatment for migraine or to correct strabismus. An international research team from the Paul Scherrer Institute, Utrecht University and the pharmaceutical company UCB has now taken an important step towards understanding the action of botulinum neurotoxin A. They have determined the x-ray crystal structure of a protein complex which clearly shows how the toxin molecule binds to the protein receptor, synaptic vesicle protein 2. The findings may prove useful for the development of improved botox drugs with a lower risk of overdosage. The structure was determined at the Swiss Light Source synchrotron at the Paul Scherrer Institute. The findings are to be published in the renowned scientific journal Nature.
The consumption of spoiled tinned food can lead to botulism in man, an intoxication that causes life-threatening paralysis. One of the causative factors is the toxin botulinum neurotoxin A which is produced by the bacterium Clostridium botulinum. It can only replicate in the oxygen-free atmosphere of the tin. The toxin attacks the neurons and prevents the passing on of neuronal signals to the muscles. In recent decades, increasingly practical applications of the toxin have been developed. Its use in cosmetics where the substance is called botox is particularly well known. When injected subcutaneously the toxin leads to relaxation of muscles and makes wrinkles disappear temporarily. This agent is also frequently used in medicine to treat migraine. In people suffering from strabismus, botulinum neurotoxin A can be used specifically to slightly weaken the eye muscle and facilitate normal vision.
Synchrotron rays reveal the protein complex structure
One fundamental step, needed to trigger the action of the botulinum toxin, is the binding of a toxin molecule to a molecule of the protein receptor synaptic vesicle protein 2 of the nerve cell. The interaction between the receptor and botulinum neurotoxin A molecule leads to a cascade of events which prevent the neuron from releasing messenger substances that normally encourage muscle movement. An international team headed by Richard Kammerer at the Laboratory of Biomolecular Research at the Paul Scherrer Institute has now succeeded in determining the exact details of the molecular interaction between botox and its receptor. Our findings are an important step towards understanding the mode of action of botulinum neurotoxin A. I am, therefore, confident that our structure will evoke major interest in the field
, explains Kammerer. For the determination of the structure the researchers used the protein crystallography method. This involves the production of large amounts of the molecules and their arrangement in a regular structure, a crystal. This crystal is then irradiated with x-rays from the Swiss Light Source synchrotron. The principle behind this technology is that x-rays are diffracted by the molecules in the crystal. From these diffraction patterns the researchers can then determine the atomic structure of the molecule under investigation.
New drugs possible
The findings not only help us to better understand the action of botox but may also be of considerable practical benefit. As a drug, botulinum neurotoxin A has a very narrow therapeutic window
, explains Roger Benoit, researcher at PSI and the first author of the article. This means that even a minor overdose can have a damaging effect
. With our findings it should be possible to develop drugs with weaker action where the risk of overdosage would then be lower.
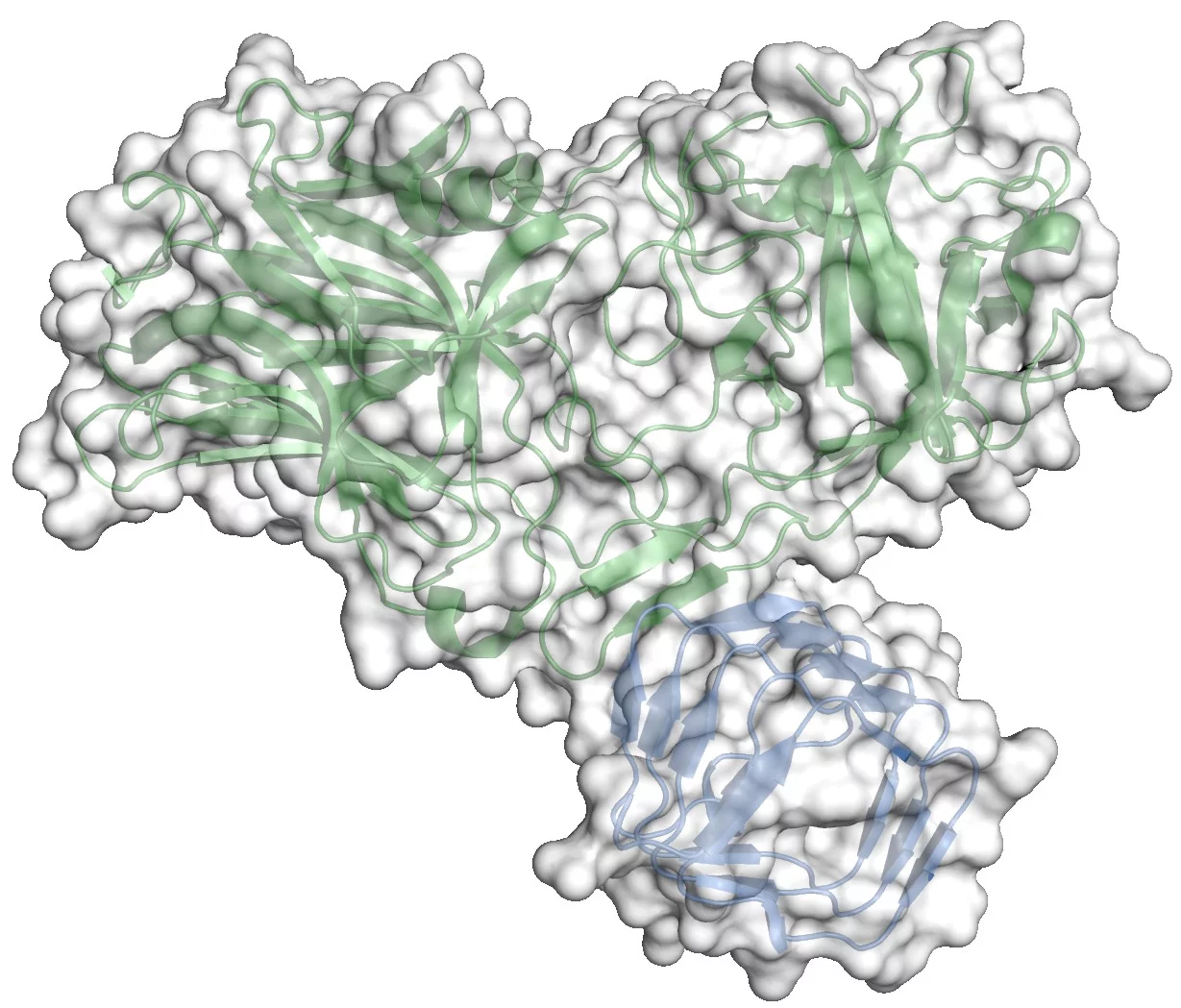
The crystal structure of the complex between the receptor binding domain of botulinum neurotoxin A (BoNT/A-RBD, shown in green) and the luminal domain of its protein receptor synaptic vesicle glycoprotein 2 (SV2C-LD, shown in blue) reveals molecular details how the toxin actually binds to SV2C-LD. The stick models in the magnification represent the amino acids at the toxin-receptor interface. The dotted lines show hydrogen bonds. The amino acids, which appear later in the film on the inner side of SV2C-LD, are phenylalanines which form the inner core of the receptor domain.
About PSI
The Paul Scherrer Institute develops, builds and operates large, complex research facilities, and makes them available to the national and international research community. The Institute's own key research priorities are in the investigation of matter and material, energy and the environment; and human health. PSI is Switzerland's largest research institution, with 1500 members of staff and an annual budget of approximately 300 million CHF.
Contact
Richard A. Kammerer PhD, Laboratory of Biomolecular Research, Paul Scherrer Institute, 5232 Villigen PSI, SwitzerlandPhone: +41 (0)56 310 4765; Email: richard.kammerer@psi.ch
Original Publication
Structural basis for recognition of synaptic vesicle protein 2C by botulinum neurotoxin ARoger M. Benoit, Daniel Frey, Manuel Hilbert, Josta T. Kevenaar, Mara M. Wieser, Christian U. Stirnimann, David McMillan, Tom Ceska, Florence Lebon, Rolf Jaussi, Michel O. Steinmetz, Gebhard F.X. Schertler, Casper C. Hoogenraad, Guido Capitani and Richard A. Kammerer
Nature Advance Online Publication 17 November 2013
DOI: 10.1038/nature12732