Tender X-rays show how one of nature’s strongest bonds breaks
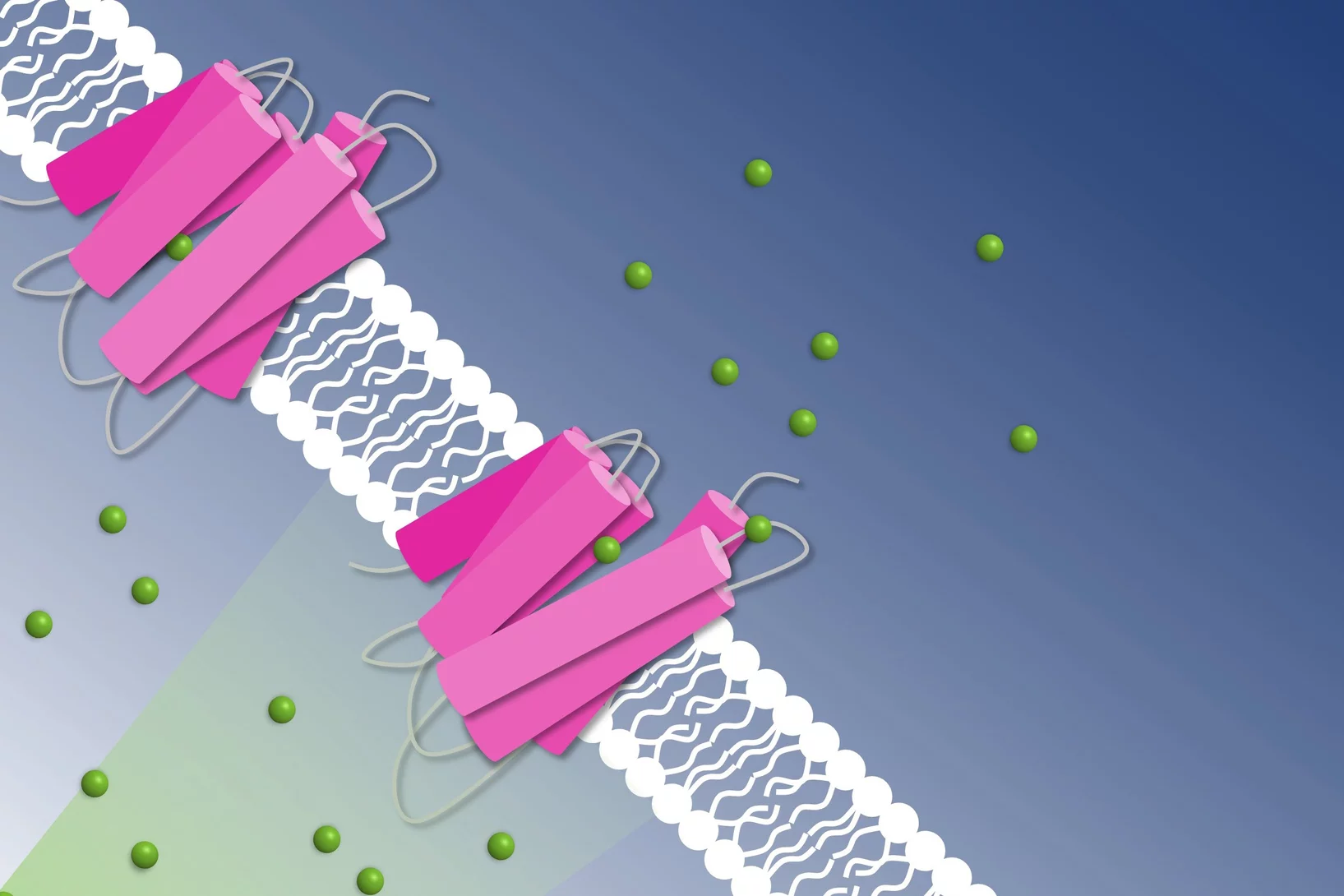
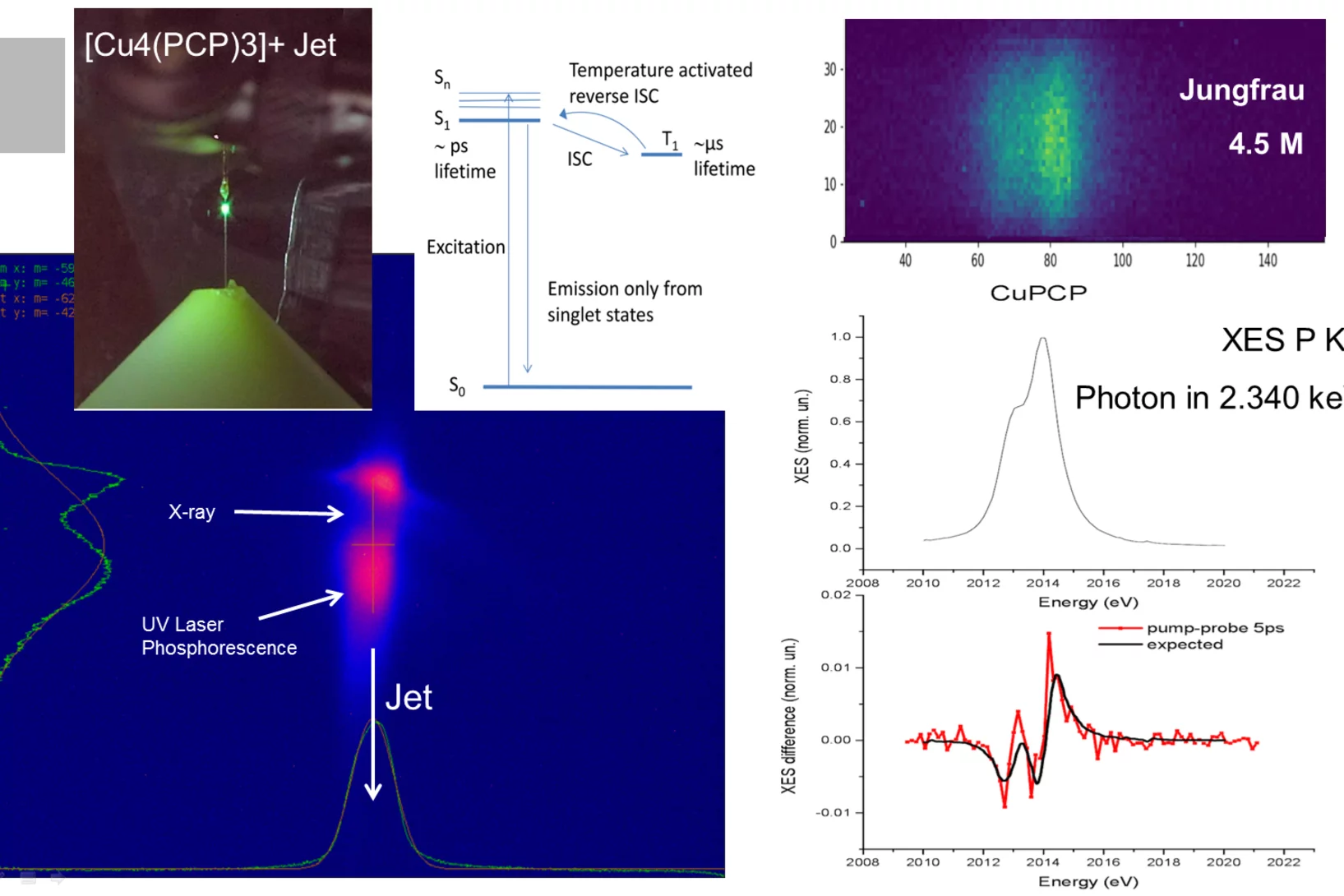
Short flashes of an unusual kind of X-ray light at SwissFEL and SLS bring scientists closer to developing better catalysts to transform the greenhouse gas methane into a less harmful chemical.
How to get chloride ions into the cell
A molecular movie shot at PSI reveals the mechanism of a light-driven chloride pump
EU XFEL Young Scientist Award for Camila Bacellar
Camila Bacellar, beamline scientist and group leader of the Alvra endstation at SwissFEL, has received the European XFEL Young Scientist Award. The award recognises the contribution of young scientists to research at the European XFEL.
Déformation inattendue d’une protéine
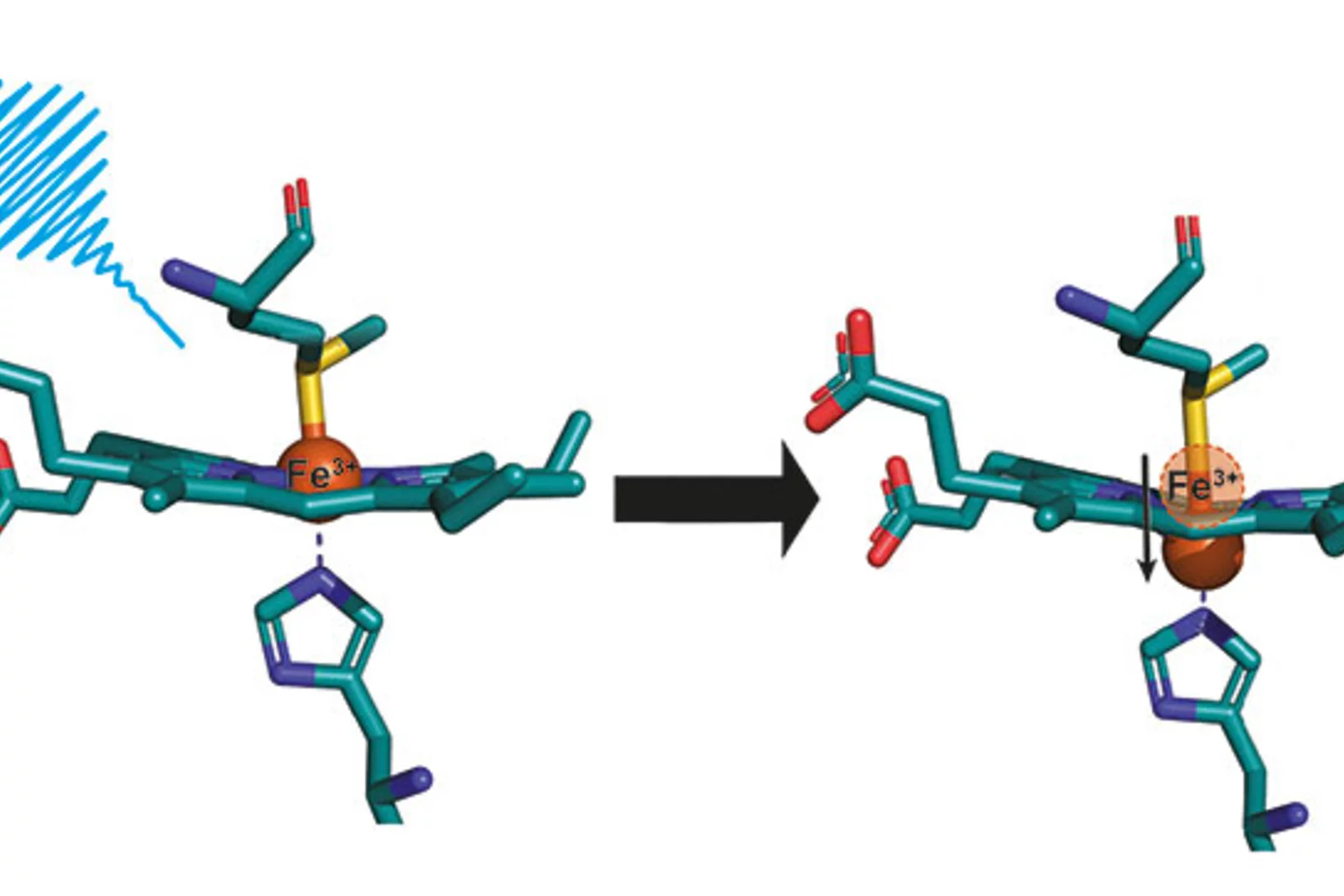
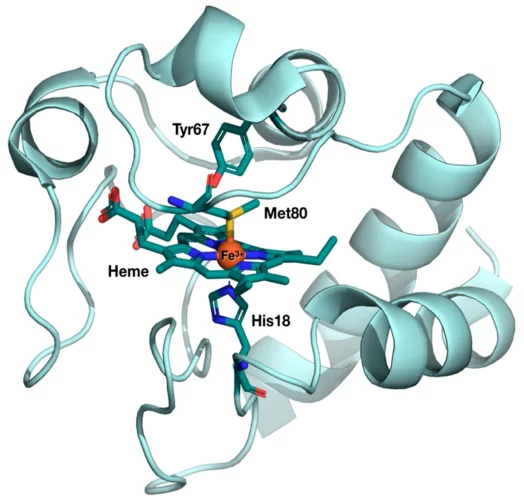
Des chercheurs du PSI ont découvert un secret du cytochrome c, que cette protéine vitale avait bien gardé jusque-là. Des mesures au laser à rayons X à électrons libres SwissFEL ont mis en évidence des modifications structurelles que la science avait pourtant exclues pour ce type de biomolécules.
Advances in de novo protein structure determination using long-wavelength native-SAD phasing at SwissFEL
An international team of scientists from the Paul Scherrer Institute and members of the LeadXpro and Heptares pharmaceutical companies led by Karol Nass (Alvra group, SwissFEL) demonstrated a significant advancement in de novo protein structure determination at X-ray free-electron lasers. Their article, published recently in IUCrJ (DOI: 10.1107/S2052252520011379), describes structure determination of a membrane protein and an important drug target (A2A adenosine receptor) by native single-wavelength anomalous diffraction (native-SAD) at SwissFEL with up to ten fold reduction in the required number of indexed images.
Unraveling the structural dynamics of Heme proteins at SwissFEL
The results from the very first user experiment at SwissFEL have just been published in the Proceedings of the National Academy of Sciences (PNAS). The measurements probed the electron transport properties of the cytochrome c protein, which is found in cellular mitochondria. The measurements show that when the Fe atom at the centre of the protein undergoes electronic excitation, for example when it gains or loses and electron, the active centre of the protein undergoes a doming structural rearrangement. This result raises interesting questions about how this structural change is involved in the electron transfer properties of cytochrome c.
Elucidation du mécanisme d’une pompe à sodium contrôlée par la lumière
Des chercheurs de l’Institut Paul Scherrer PSI ont réussi une première: réaliser des prises de vue d’une pompe à sodium en action, plus précisément d’une pompe à sodium de cellules bactériennes contrôlée par la lumière. Ces éléments de connaissance sont prometteurs pour le développement de nouvelles méthodes dans le domaine de la neurobiologie. Pour leurs analyses, les chercheurs ont utilisé le nouveau laser à rayons X à électrons libres SwissFEL.
A la recherche du matériau électroluminescent du futur
A l’Institut Paul Scherrer PSI, des chercheurs ont scruté l’intérieur d’un matériau prometteur pour les diodes organiques électroluminescentes (OLED). Leurs conclusions contribueront au développement de matériaux électroluminescents à très bon rendement lumineux et peu coûteux à la fabrication.
First serial femtosecond crystallography experiment using SwissFEL’s large bandwidth X-ray pulses
The typical mode of operation at XFEL facilities uses the so-called self-amplified spontaneous emission (SASE) process to generate the short, bright X-ray pulses. This mode of operation is stochastic in nature, causing some variance in intensity and spectrum on a shot-to-shot basis, which makes certain types of crystallographic measurements much more challenging.
Towards X-ray Transient Grating Spectroscopy at SwissFEL
The high brilliance of new X-ray sources such as X-ray Free Electron Laser opens the way to non-linear spectroscopies. These techniques can probe ultrafast matter dynamics that would otherwise be inaccessible. One of these techniques, Transient Grating, involves the creation of a transient excitation grating by crossing X-ray beams on the sample. Scientists at PSI have realized a demonstration of such crossing by using an innovative approach well suited for the hard X-ray regime.
First femtosecond protein pump-probe measurements at SwissFEL
A major milestone in the commissioning of SwissFEL has been reached: the first pump-probe experiments on proteins have been successfully carried out. Crystals of several retinal-binding proteins were delivered in a viscous jet system and a femtosecond laser was used to start the isomerization reaction. Microsecond to sub-picosecond snapshots were then collected, catching the retinal proteins shortly after isomerization of the chromophore.
Demonstration of femtosecond X-ray pump X-ray probe diffraction on protein crystals
Our experiments, published in the September issue of Structural Dynamics, demonstrate the feasibility of time-resolved pump-multiprobe X-ray diffraction experiments on protein crystals using a split-and-delay setup which was temporarily installed at the LCLS X-ray Free Electron Laser.
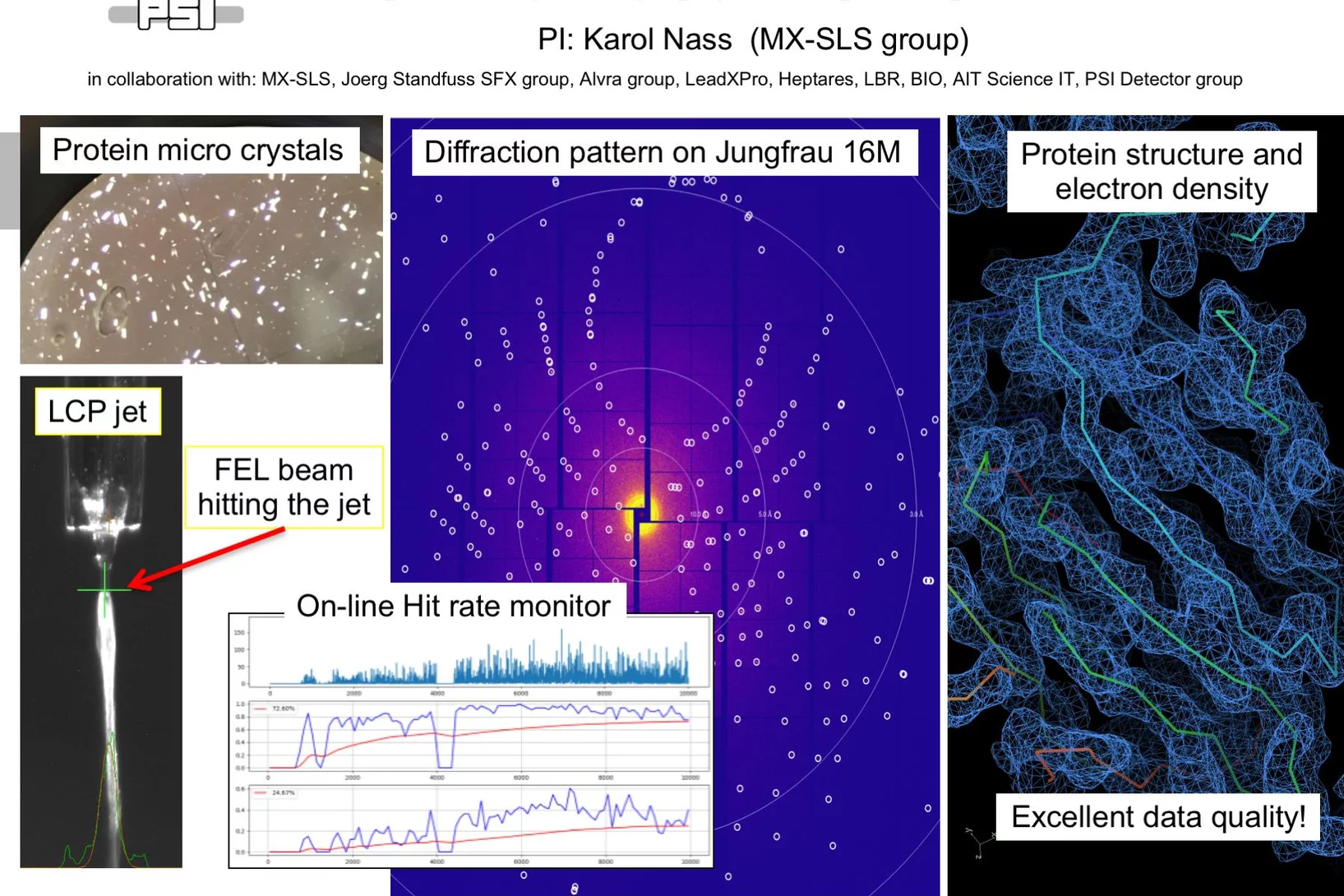
First serial femtosecond crystallography (SFX) pilot user experiment at SwissFEL
On the 7th to 12th of August 2018, a collaborative group of scientists from the Paul Scherrer Institute and members of the LeadXpro and Heptares pharmaceutical companies led by Karol Nass (PSI macromolecular crystallography MX-SLS group) performed the first serial femtosecond crystallography (SFX) pilot user experiment at the SwissFEL X-ray free electron laser (XFEL).
Capteur biologique de lumière filmé en pleine action
Une équipe de chercheurs de l'Institut Paul Scherrer PSI a enregistré, à l'aide d'un laser à rayons X, l'un des processus les plus rapides en biologie. Le film moléculaire ainsi réalisé révèle la manière dont le capteur de lumière rétinal est activé dans une molécule de protéine. Des réactions de ce type interviennent dans de nombreux organismes. Ce film montre pour la première fois comment une protéine pilote de manière efficace la réaction du capteur de lumière intégré en son sein.
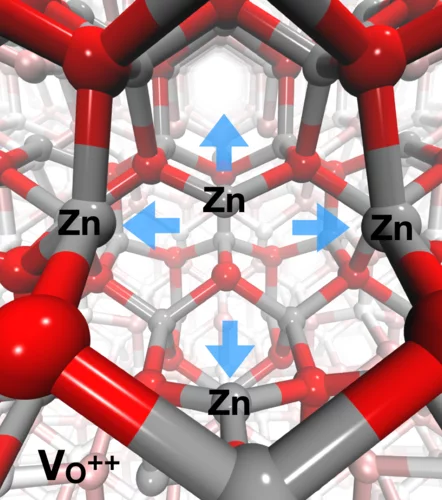
Identification of hole trapping sites in ZnO nanomaterials
Members of the Alvra group led an investigation into the fate of charge carrier dynamics in metal oxide semiconductor nanomaterials. The experiments were performed at the Advanced Photon Source (Argonne, IL, USA) and used a PSI-designed von Hamos geometry X-ray emission spectrometer that was constructed for the experiment to perform resonant XES measurements on a solution of 32 nm diameter ZnO nanoparticles photo-excited with 3.2 eV (355 nm) short laser pulses. The measurement showed that the hole-trapping takes place within less than 100 ps and the trapping site in the ZnO crystal lattice is at oxygen vacancies in the lattice. The trapping of the hole results in a local structural distortion, where the four neighbouring Zn atoms move away from the vacancy. The measurement demonstrated the strength of the RXES technique's ability to probe both the electronic and geometric structure of materials and the results were recently published in Nature Communications.
First Pilot Experiment at SwissFEL-Alvra: UV photo-induced charge transfer in OLED system
On the 17th of December 2017 SwissFEL saw its first pilot experiment in the Alvra experimental station of the SwissFEL ARAMIS beamline.
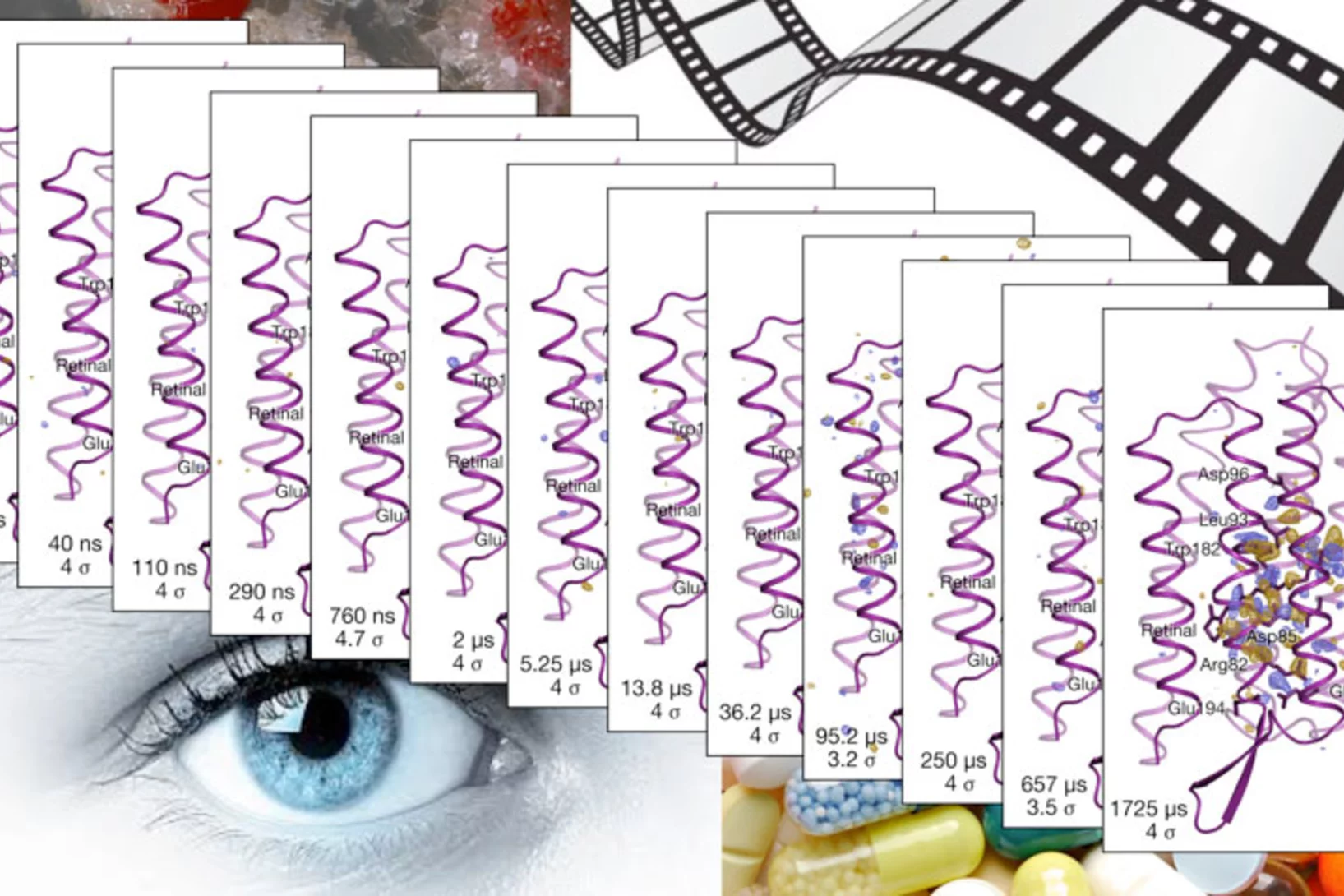
A three-dimensional movie of structural changes in bacteriorhodopsin
Snapshots of bacteriorhodopsinBacteriorhodopsin is a membrane protein that harvests the energy content from light to transport protons out of the cell against a transmembrane potential. Nango et al. used timeresolved serial femtosecond crystallography at an x-ray free electron laser to provide 13 structural snapshots of the conformational changes that occur in the nanoseconds to milliseconds after photoactivation. These changes begin at the active site, propagate toward the extracellular side of the protein, and mediate internal protonation exchanges that achieve proton transport.
L’union fait la force
Décrypter les molécules au SwissFEL et à la SLSLes protéines sont un objet de recherche convoité, mais récalcitrant. Leur étude est aujourd’hui facilitée par une nouvelle méthode développée à l’aide d’un laser à rayons X à électrons libres comme le futur SwissFEL du PSI. Elle consiste à exposer à intervalles rapprochés de petits échantillons identiques de protéines à de la lumière de type rayons X. On contourne ainsi un problème majeur auquel la recherche sur les protéines s’est heurtée jusqu’ici: produire des échantillons de taille suffisante.
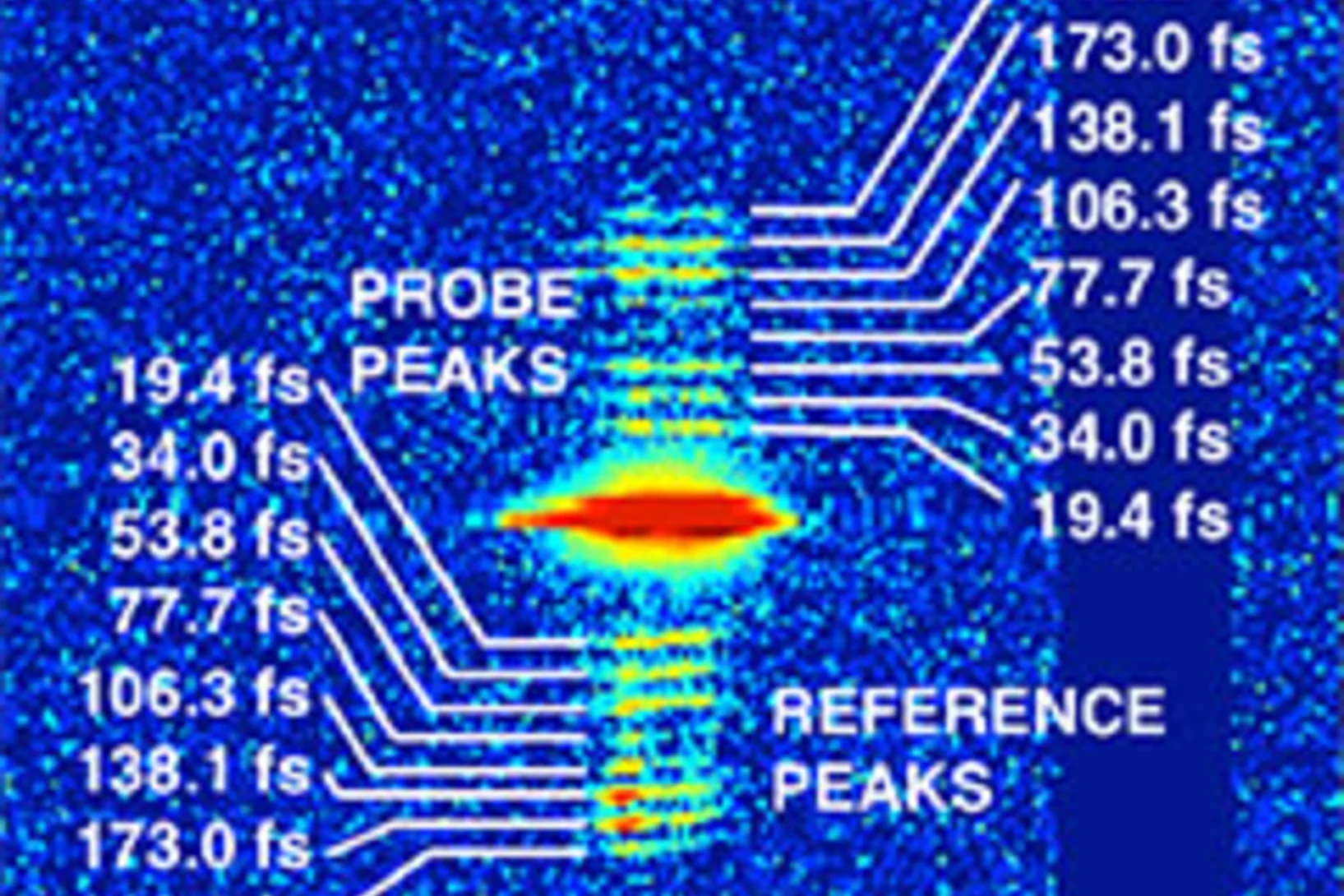
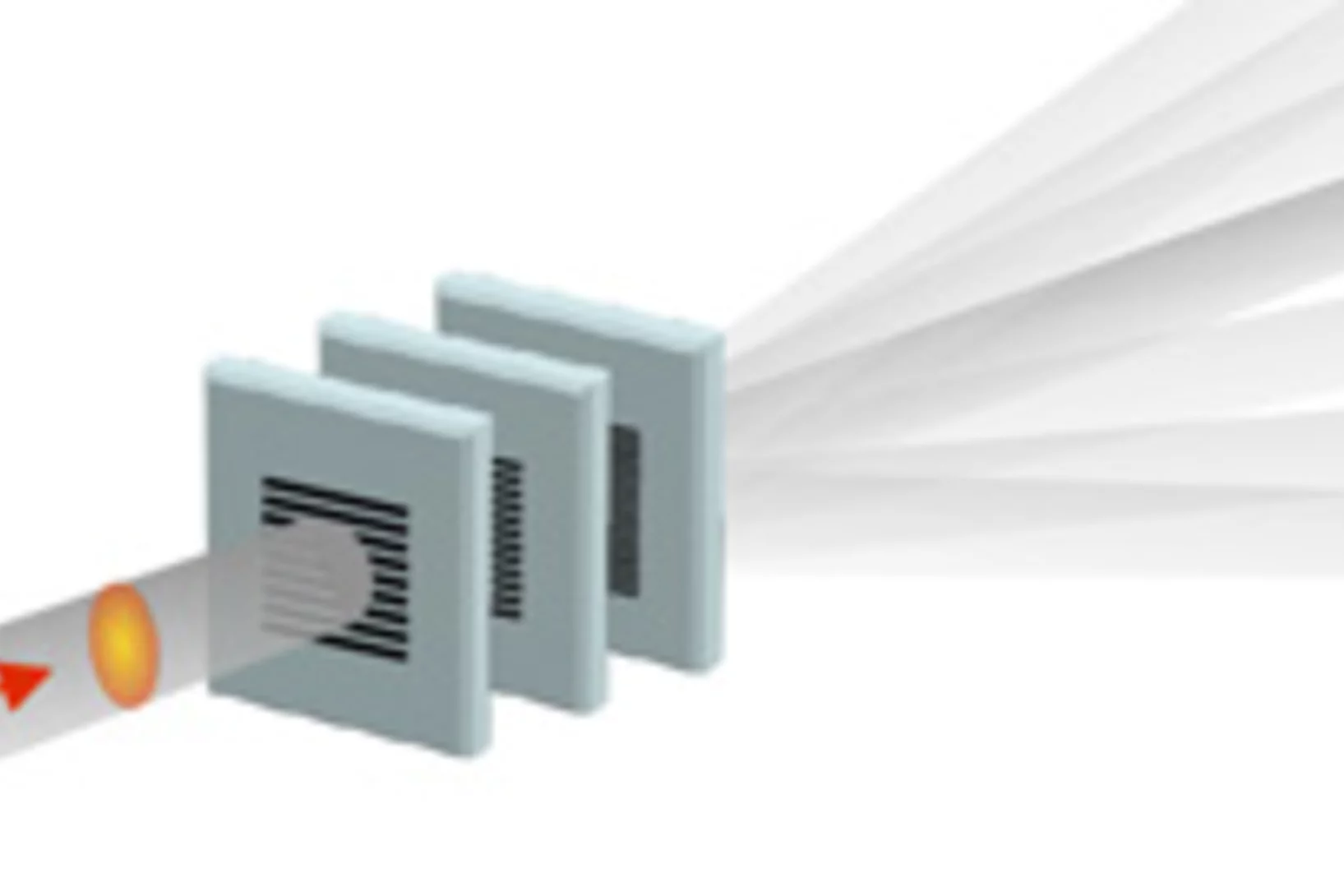
Fractionner une impulsion de rayons X pour visualiser des processus ultra rapides
Le laser à rayons X SwissFEL du PSI permettra de visualiser les différentes étapes de processus très rapides. Un nouveau procédé devrait rendre possibles des expériences encore plus précises : il consiste à fractionner chaque impulsion de rayons X, et à faire en sorte que chaque fraction de l’impulsion atteigne l’une après l’autre l’objet étudié. Le principe de ce processus rappelle celui de l’ancienne chronophotographie.