Fuel cells – electricity from hydrogen and oxygen
A fuel cell generates electricity from the conversion of hydrogen and oxygen. In addition to electricity, heat and water are also produced as end-products. In principle, the chemical processes correspond here to the so-called explosive gas reaction, in which a mixture of oxygen and hydrogen is made to explode. In a fuel cell, however, the energy released does not go up in smoke, but is converted into electrical energy.
Fuel cells can be used as compact and transportable sources of power in electric cars, as well as, for example, in electronic devices or residential homes. Fuel cells do not generate harmful exhaust emissions where they are being used and can therefore help to keep the air in cities clean. If the hydrogen is also generated by means of non-fossil primary energy sources – such as solar energy – the fuel cell produces clean energy that is low in CO2 emission.
PSI has been engaged for many years at all levels of fuel-cell research, from the optimisation of individual components to the development of complete fuel-cell systems. A special feature of research at PSI is the intensive use of investigation methods at PSI's large-scale facilities, which enable unique insights to be obtained into the inner workings of fuel cells and the materials used in them. As a result, scientists can gain a basic understanding of the processes that take place in the cell and improve it in very selective ways.
Contribution to the energy future
Fuel cells could make an important contribution to the energy system of the future. This is set to be overhauled step by step until 2050. An important aspect of the federal government's package of measures formulated in the Energy strategy 2050
is the expansion of photovoltaic and wind power plants. However, they produce variable amounts of electricity depending on weather conditions, time of day and season. Ways must therefore be found to integrate them flexibly into the energy system.
At PSI, researchers are exploring the potential of fuel cells in this respect. If photovoltaic or wind power plants generate too much electricity, their energy can be stored temporarily in hydrogen and then be converted back into electricity by fuel cells upon demand.
In order to determine the potential of fuel cells for integrating surplus power, researchers are investigating how fuel cells behave in an industrial operating environment aligned towards this scenario. Since 2016, an experimental platform has been available to them at PSI, the ESI platform (ESI stands for Energy System Integration
). It enables researchers to simulate an industrial environment on a small scale.
How does a fuel cell work?
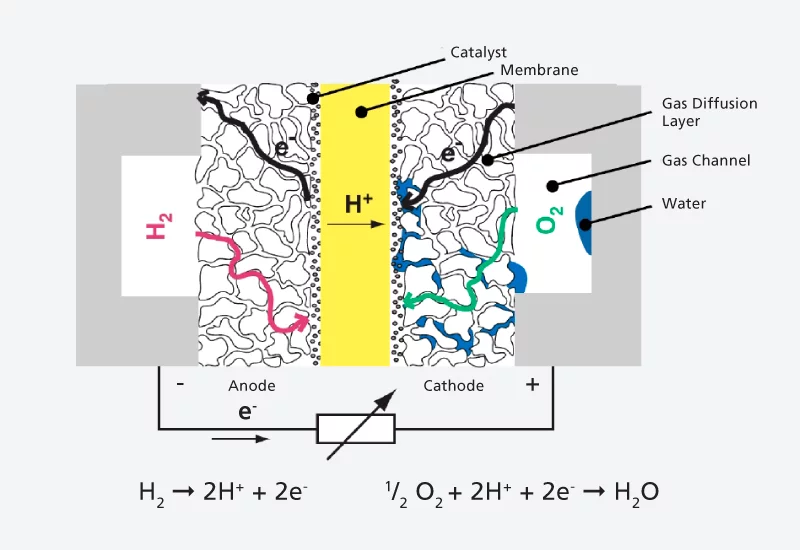
In a polymer-electrolyte fuel cell, such as that which is the subject of research at PSI, oxygen and hydrogen are separated by a thin film, or membrane. This membrane only allows the atomic nuclei of hydrogen, i.e. protons, to pass through. The electrons have to take a detour via an electric circuit. Here they act as an electric current, which can be used, for example, to power a vehicle. A fuel cell of this kind consists of several layers, the middle one of which is the membrane. On both sides of the membrane is a thin layer of catalyst material, which makes the chemical reactions possible, and on the outer side of each catalyst layer is a gas diffusion layer. This consists of a carbon-fibre fleece, which has to perform several functions at once – transporting the reaction gases to the membrane, removing the resulting water on the oxygen side
and carrying the resulting electric current.
Understanding processes – optimising cells
Although fuel cells today are being mass produced and their various components are commercially available, the detailed processes taking place in the cells are in many cases not yet fully understood. One objective of research at PSI is therefore to determine the influence which the characteristics of the different materials used has on processes in a fuel cell and, in particular, to identify the processes which inhibit the efficiency of a cell. These studies enable materials for use in fuel cells to be optimised and thereby the performance of future generations of fuel cells to be raised.
Watching the water
One process that is being intensively studied at PSI is the behaviour of water in a fuel cell. It must be borne in mind that water plays many roles here: it is produced in the reaction of hydrogen and oxygen and has to be efficiently removed afterwards, because otherwise it clogs the pores of the gas diffusion layer and prevents the reaction gases from reaching the catalyst. At the same time, however, the membrane must not dry out, because it then becomes less efficient at conducting protons – so there always has to be an optimal quantity of water present. The test methods available at PSI now make it possible to directly monitor the flow of water in a fuel cell. Tomography at the Swiss Light Source SLS, for example, shows in detail how water behaves between the fibres of the gas diffusion layer, the way it flows through the layer or whether it forms drops in the pores. With the aid of neutron radiography, on the other hand, the patterns of water flow can be visualized in a whole functional cell – and this despite the fact that the water is behind a thick metal housing.
Numerous research topics
Research on fuel cells at PSI is very diverse - some examples are the optimisation of catalytic layers, the development of novel processes for the production of more stable and less expensive catalysts and membranes, and the investigation of gas flows through the diffusion layer. These are important research goals that improve the service life and efficiency of fuel cells and reduce costs.
PSI's commitment extends to the development of fuel cell systems for specific applications such as those tested on the ESI platform for storing surplus power. Researchers thereby work closely with industry partners.
Related information
- Research with neutrons and synchrotron light
Research possibilities on the large research facilities providing insights into fuel cells. - Electrochemistry Laboratory
This is the place for the PSI fuel cell research. - ESI Platform