Researchers at the Paul Scherrer Institute and the University of Basel deliberately alter the properties of a porphyrin molecule, with many possible technological applications
As a component of the haemoglobin molecule, porphyrin enables the transport of oxygen within the human body. However, in a slightly altered form, it may find also various applications in technical devices. A cobalt atom introduced into a porphyrin molecule acts as a tiny magnet, which aligns itself with the orientation of the magnetization of the substrate to which the molecule is attached. Researchers at the Paul Scherrer Institute and the University of Basel have shown that this property can be switched on and off by means of a simple chemical procedure, so that the molecule can be used as a tiny molecular switch. Even though this is still at the stage of fundamental research, it is already possible to think of a whole variety of applications for this process, such as in magnetic data storage devices or even in quantum computers. The researchers report on their work in the journal Nature Communications.
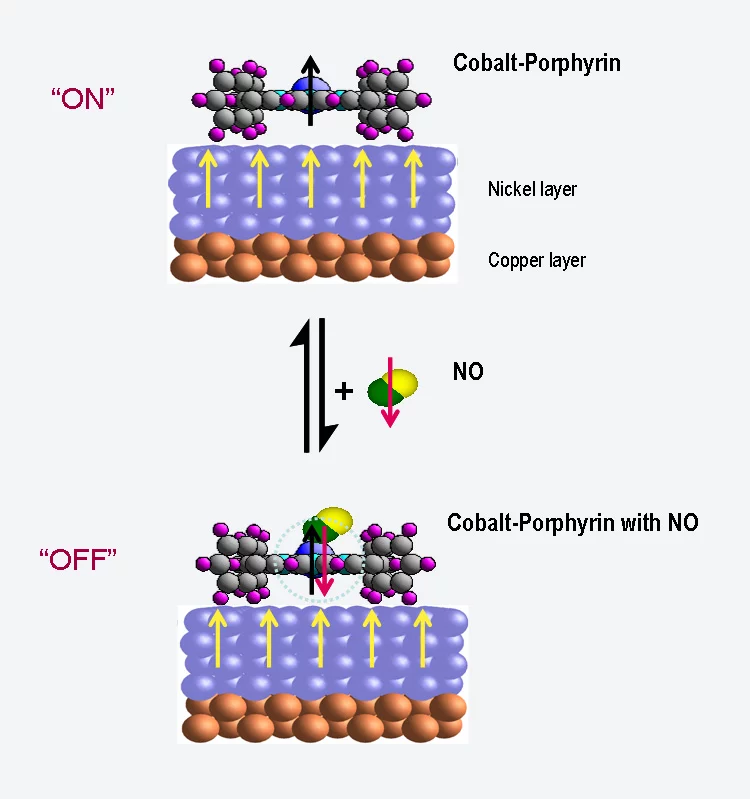
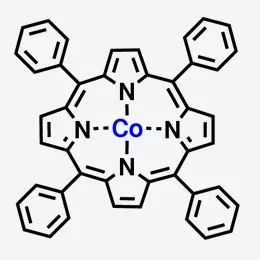
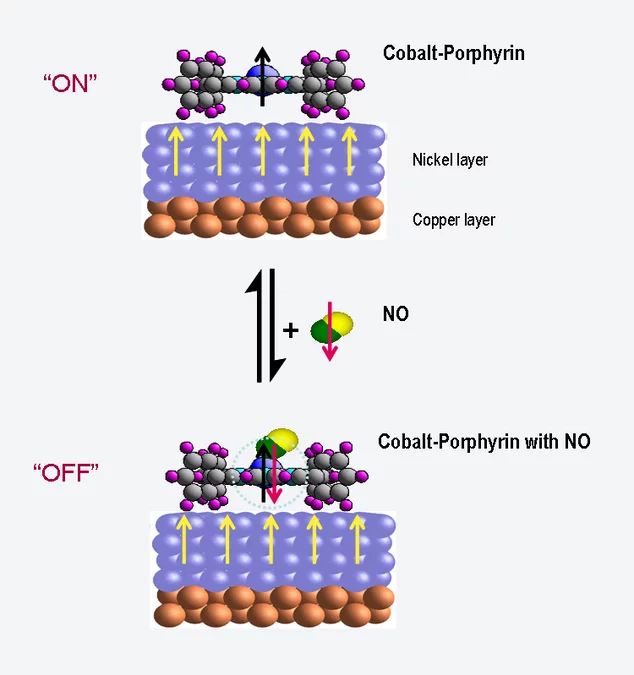
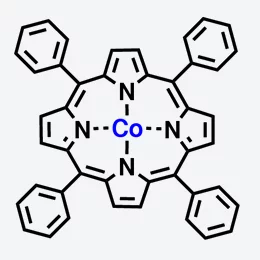
In the human body, haemoglobin bonds with oxygen and transports it in the blood to the places where it is needed. A haemoglobin molecule is made up of four porphyrin molecules, each of which contains an iron atom that can bond to oxygen. Yet porphyrin molecules can also have interesting magnetic properties, as experiments with slightly altered molecules have shown, in which the iron has been replaced by cobalt. In this case, the cobalt atom behaves as a tiny magnet – physicists say that it carries a magnetic moment. If this molecule is attached to a magnetic surface, the orientation of the magnetic moment aligns itself to the direction of magnetization of the surface. Thus, the molecule acts as a magnetic switch, an effect that has already been described by researchers from the Paul Scherrer Institute (PSI) in 2005.
Switching magnetism on and off
Now, the same research group has shown that this magnetic switching capability can itself also be switched on and off. They have shown that the cobalt atoms become non-magnetic when they are bonded with a molecule of nitric oxide (NO). But when the nitric oxide is released through heating, the cobalt again becomes magnetic. Until now, we have only investigated a large group of molecules”
, explains Nirmalya Ballav, who had the idea for the experiment, but, based on our results, one can envisage that it will become possible to induce this process in single molecules
. This could be used, perhaps, for data storage; in order to store a bit of information, where one would use one of the two states reacts to magnetization
or does not react to magnetization
of a single molecule or a small group of molecules. In this way, a stable storage device could be created that could be erased simply by warming. And, as a single porphyrin molecule is only about one nanometre in size, data could be stored much more densely than is possible today.
Futuristic ideas
However, many other applications in many other fields are also conceivable. It may be possible, for example, to use porphyrin molecules to create the exotic quantum states needed for quantum computers. They may also help us to understand the processes taking place within the spintronic components that are becoming more and more common in electronic devices such as read-and-write heads for computer hard disks. Another possibility could be to coat glass with a layer of porphyrin molecules and to use the fact that these molecules, and consequently their optical properties, can be changed. That this kind of molecule can actually change its appearance when brought into contact with another substance is something we know from everyday life: when the porphyrin in our blood is loaded with oxygen, our lips appear red, otherwise they look blue. We need another ten years to find out which applications can actually be implemented successfully
, says Thomas Jung, head of the research group Molecular Nanoscience at the Paul Scherrer Institute and professor at the University of Basel, to curb excessive expectations, and explains, Our research is performed with a view to developing future technologies, but I do not know yet which technologies will establish themselves, based on this effect, this new way of controlling magnetism.
The experiment
In their experiment, the researchers attached the porphyrin molecules to a magnetized nickel surface. At first, they observed that the magnetic moments of the cobalt atoms aligned themselves with the magnetization of the nickel substrate, and followed it when it was changed. When the porphyrin layer was exposed to nitric oxide, however, the effect vanished – the cobalt became non-magnetic. These measurements were performed at the Swiss Light Source SLS of the Paul Scherrer Institute. Here, it is not only possible to measure the magnetization of a material but also to determine the particular chemical element responsible for it. Thus, one can distinguish whether the observed magnetization stems from the nickel or the cobalt.
The Team
Dorota Chylarecka and Kathrin Müller (physicists) und Christian Wäckerlin (a nanoscientist) performed the experiments as Ph.D. students supported by the Swiss National Science Foundation and the Argovia Network Nanotechnology of the Canton of Aargau. Cristian Iacovita, from the University of Basel, developed the necessary expertise for the investigation of such molecules using scanning tunnelling microscopy. Frithjof Nolting and Armin Kleibert, experts in the field of atom-sensitive analysis of magnetization, are responsible for the beamline at the Swiss Light Source SLS at which the experiments were performed. The idea for the experiment came from Nirmalya Ballav, a physico-chemist from India who has spent many years working at Heidelberg University. The experiment is an excellent example of the combination of the expertise of PSI and of Thomas Jung’s research group in the national research network of the Swiss Nanoscience Institute.
Text: Paul Piwnicki
About PSI
The Paul Scherrer Institute develops, builds and operates large-scale, complex research facilities, and makes these facilities available to the national and international research community. The Institute’s own research focuses on solid-state physics and the materials sciences, elementary particle physics, biology and medicine, as well as research involving energy and the environment. With a workforce of 1300 and an annual budget of about 260 million CHF, PSI is the largest research institution in Switzerland.
About SNI
The Swiss Nanoscience Institute (SNI) emerged from the National Research Focus (NFS) Nanosciences initiative founded in 2001, and constitutes a University Research Priority Programme. In the SNI, fundamental research is strongly linked to application-oriented research. Within various projects, scientists are concerned with structures in the nanometre range. Their goal is to stimulate innovation for the life sciences, for sustainability, and for information and communication technology. The Department of Physics at the University of Basel is acting as “Leading House” and is coordinating the network formed by universities and research institutes together with their industrial partners as well as the Argovia Network, initiated by the Canton of Aargau in 2006.
Contact
Prof. Dr. Thomas Jung, Laboratory for Micro- and Nanotechnology,Paul Scherrer Institute, 5232 Villigen PSI, Switzerland,
Phone: +41 56 310 45 18 oder +41 61 267 39 11, E-Mail: Thomas.Jung@psi.ch
Dr. Nirmalya Ballav, Laboratory for Micro- and Nanotechnology,
Paul Scherrer Institute, 5232 Villigen PSI, Switzerland,
Phone: +41 56 310 45 59, E-Mail: nirmalya.ballav@psi.ch
Dr. Paul Piwnicki, Communications Department,
Paul Scherrer Institute, 5232 Villigen PSI, Switzerland,
Phone: +41 56 310 29 40 E-Mail: paul.piwnicki@psi.ch
Original Publication
Controlling spins in adsorbed molecules by a chemical switch,Christian Wäckerlin, Dorota Chylarecka, Armin Kleibert, Kathrin Müller, Cristian, Iacovita, Frithjof Nolting, Thomas A. Jung & Nirmalya Ballav,
Nature Communications, 24 August,
DOI: 10.1038/ncomms1057
Induced magnetic ordering in a molecular monolayer,
A. Scheybal, T. Ramsvik, R. Bertschinger, M. Putero, F. Nolting and T.A. Jung,
Chem. Phys. Lett. 411, 214–220 (2005),
DOI: 10.1016/j.cplett.2005.06.017