Researchers at the Paul Scherrer Institute PSI have deciphered the molecular processes that first occur in the eye when light hits the retina. The processes – which take only a fraction of a trillionth of a second – are essential for human sight. The study has now been published in the scientific journal Nature.
It only involves a microscopic change of a protein in our retina, and this change occurs within an incredibly small time frame: it is the very first step in our light perception and ability to see. It is also the only light-dependent step. PSI researchers have established exactly what happens after the first trillionth of a second in the process of visual perception, with the help of the SwissFEL X-ray free-electron laser of the PSI.
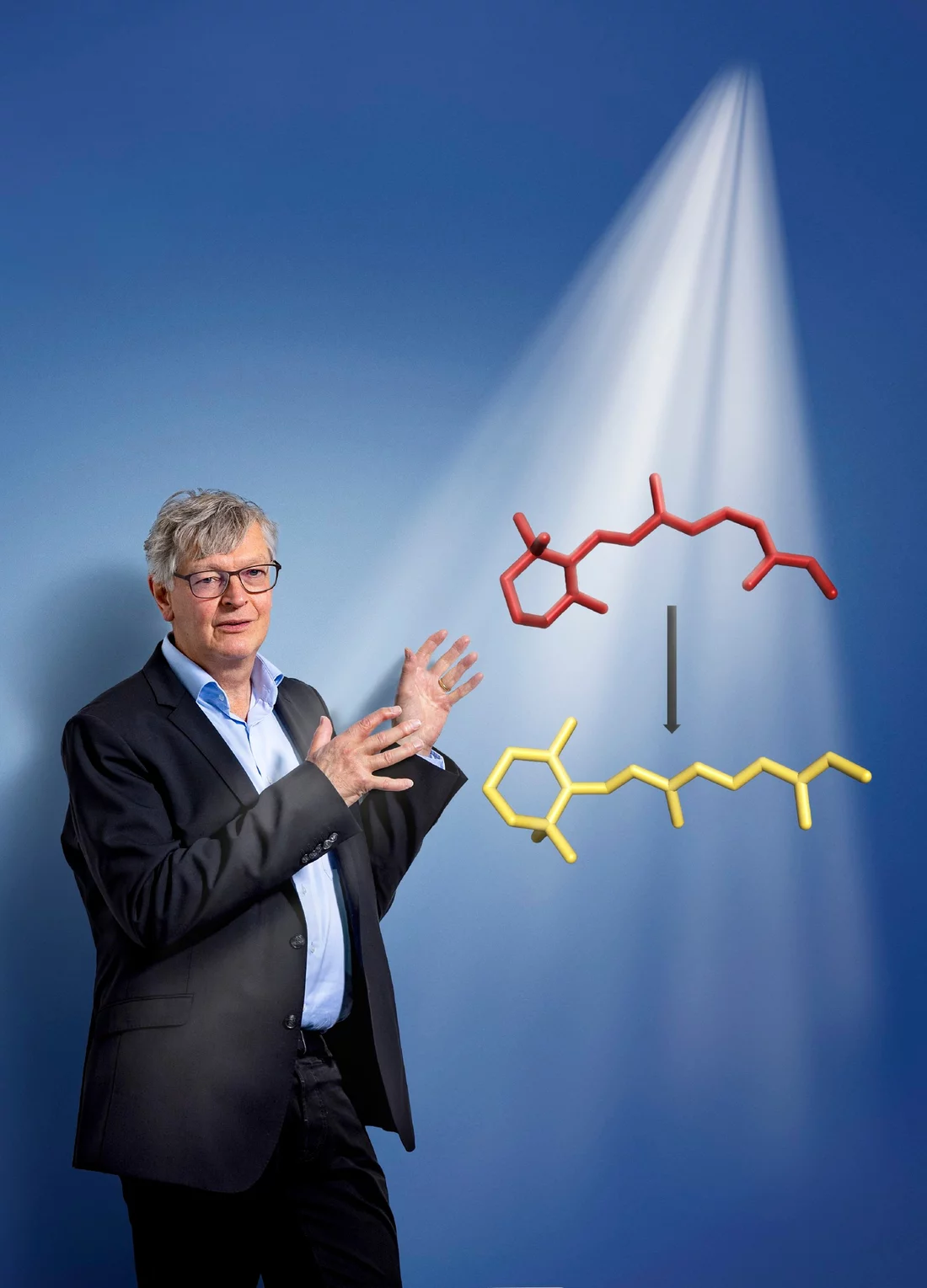
At the heart of the action is our light receptor, the protein rhodopsin. In the human eye it is produced by sensory cells, the rod cells, which specialise in the perception of light. Fixed in the middle of the rhodopsin is a small kinked molecule: retinal, a derivative of vitamin A. When light hits the protein, retinal absorbs part of the energy. With lightning speed, it then changes its three-dimensional form so the switch in the eye is changed from “off” to “on”. This triggers a cascade of reactions whose overall effect is the perception of a flash of light.
Tied – but yet free
But what happens in detail when retinal transforms from what is known as the 11-cis form into the all-trans form? “We have known about the starting point and the end product of the retinal transformation for some time, but so far no one has been able to observe in real time exactly how the change occurs in the sight pigment rhodopsin,” says Valérie Panneels, a scientist with PSI’s Biology and Chemistry Research Division.
Panneels compares the process to a cat falling back-first from a tree but somehow landing on its feet unharmed. “The question is: what states does the cat adopt during its fall as it rights itself to land on its feet?”
As the PSI scientists discovered, the “retinal cat” starts off by turning the middle of its body. For Valerie Panneels, the “eureka moment” came when she realised something else that occurs: the protein absorbs part of the light energy to briefly inflate a tiny amount – “like our chest expanding when we breathe in, only to contract again shortly afterwards.”
During this “breathing in” stage, the protein temporarily loses most of its contact with the retinal that sits in its middle. “Although the retinal is still connected to the protein at its ends through chemical bonds, it now has room to rotate.” At that moment, the molecule resembles a dog on a loose leash that is free to give a jerk.
Shortly afterwards the protein contracts again and has the retinal firmly back in its grasp, except now in a different more elongated form. “In this way the retinal manages to turn itself, unimpaired by the protein in which it is held.”
One of the fastest natural processes
The transformation of the retinal from 11-cis kinked form into the all-trans elongated form only takes a picosecond, or one trillionth (10-12) of a second, making it one of the fastest processes in all of nature.
The only way of recording and analysing such rapid biological processes is with an X-ray free-electron laser like the SwissFEL. “The SwissFEL allows us to study in detail the fundamental processes of the human body, such as vision,” says Gebhard Schertler, Head of PSI’s Biology and Chemistry Research Division and joint lead author of the study along with Valérie Panneels.
To return to the analogy of the cat, this would be like filming its fall with a high-speed camera, but with one major difference: the filming speed of the SwissFEL camera is a milliard times faster. Working with large research facilities also involves much more than simply pressing a shutter button. The PhD student Thomas Gruhl, who went on to work as a postdoc researcher at the Institute for Structural and Molecular Biology in London, has spent years developing a method of producing high-quality rhodopsin crystals capable of delivering ultra-high resolution data. Ultimately only these data made it possible to perform the necessary measurements at SwissFEL and – before the SwissFEL was built – at the X-ray free-electron laser SACLA in Japan.
This experiment once again shows SwissFEL’s vital role in Swiss research. “It will probably help us come up with many more solutions in future,” says Gebhard Schertler. “Amongst other things, we are also developing methods for investigating dynamic processes in proteins that are not normally activated by light.” The scientists use artificial means to make such molecules responsive to light: either they make appropriate changes to the binding partners or they mix proteins with binding partners in the crystal so quickly that they can be examined at the SwissFEL. In any case, the procedure involved is definitely much more complicated than simply pointing a camera at a cat falling from a tree.
Contact
Original publication
-
Gruhl T, Weinert T, Rodrigues MJ, Milne CJ, Ortolani G, Nass K, et al.
Ultrafast structural changes direct the first molecular events of vision
Nature. 2023; 615: 939-944. https://doi.org/10.1038/s41586-023-05863-6
DORA PSI
About PSI
The Paul Scherrer Institute PSI develops, builds and operates large, complex research facilities and makes them available to the national and international research community. The institute's own key research priorities are in the fields of future technologies, energy and climate, health innovation and fundamentals of nature. PSI is committed to the training of future generations. Therefore about one quarter of our staff are post-docs, post-graduates or apprentices. Altogether PSI employs 2300 people, thus being the largest research institute in Switzerland. The annual budget amounts to approximately CHF 460 million. PSI is part of the ETH Domain, with the other members being the two Swiss Federal Institutes of Technology, ETH Zurich and EPFL Lausanne, as well as Eawag (Swiss Federal Institute of Aquatic Science and Technology), Empa (Swiss Federal Laboratories for Materials Science and Technology) and WSL (Swiss Federal Institute for Forest, Snow and Landscape Research). (Last updated in June 2024)