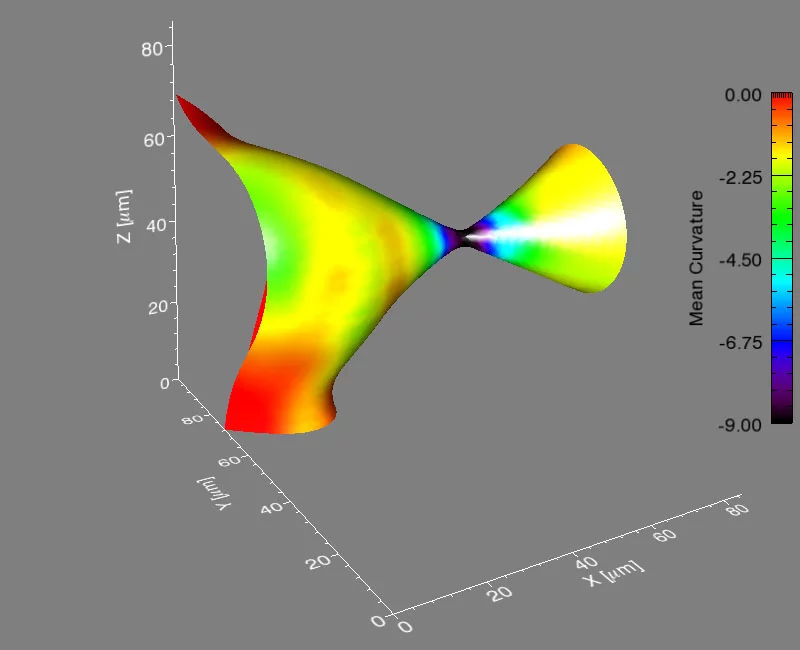
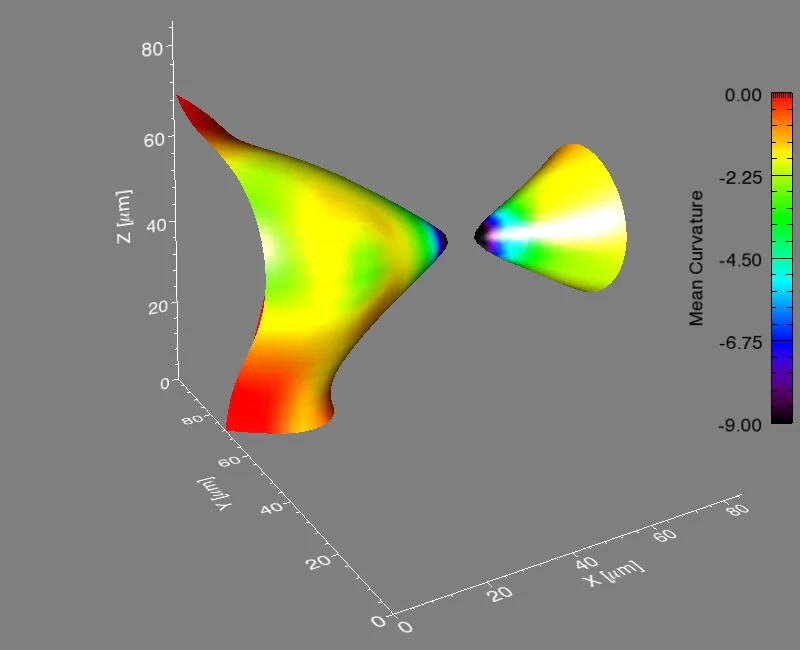
Many important materials are composed of several phases – regions that differ in structure or chemical composition. When such a material is heated, atoms move from one phase to another, which changes the distribution of the phases – and thus, the properties of the material. Researchers from Northwestern University in the USA, the Paul Scherrer Institute, and Risø National Laboratory for Sustainable Energy in Denmark have now shown that for an important case, there is a general law describing this process that is valid for all classes of materials – including metals and polymers.
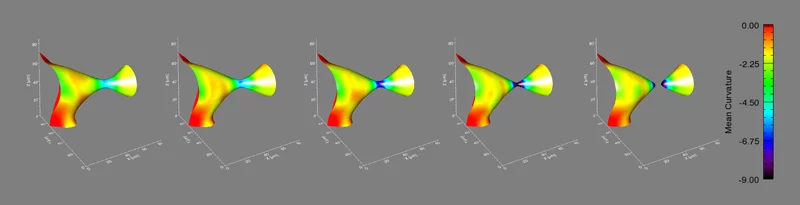
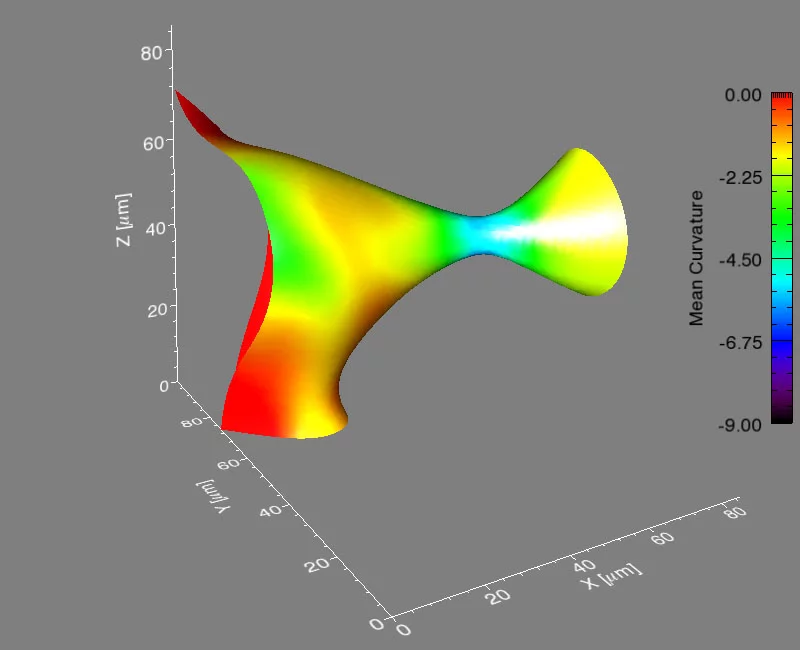
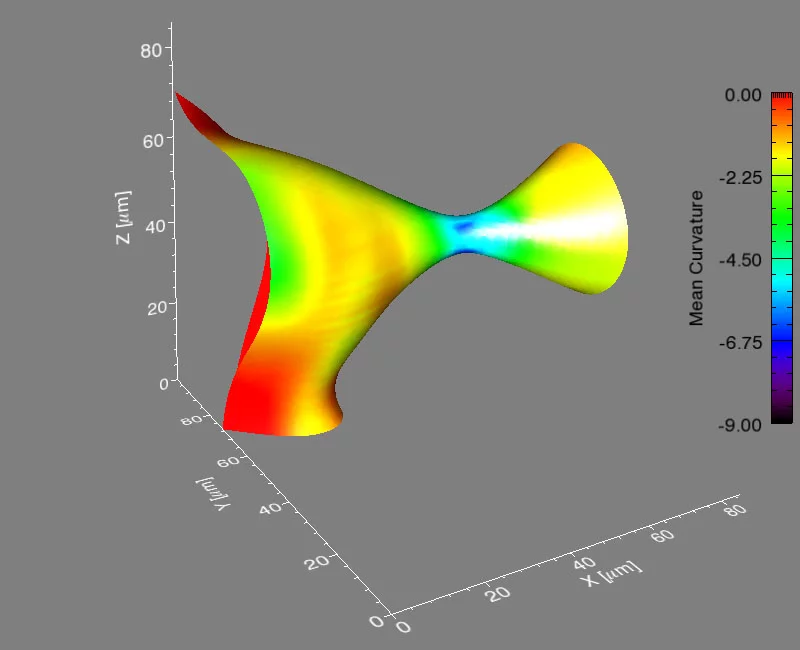
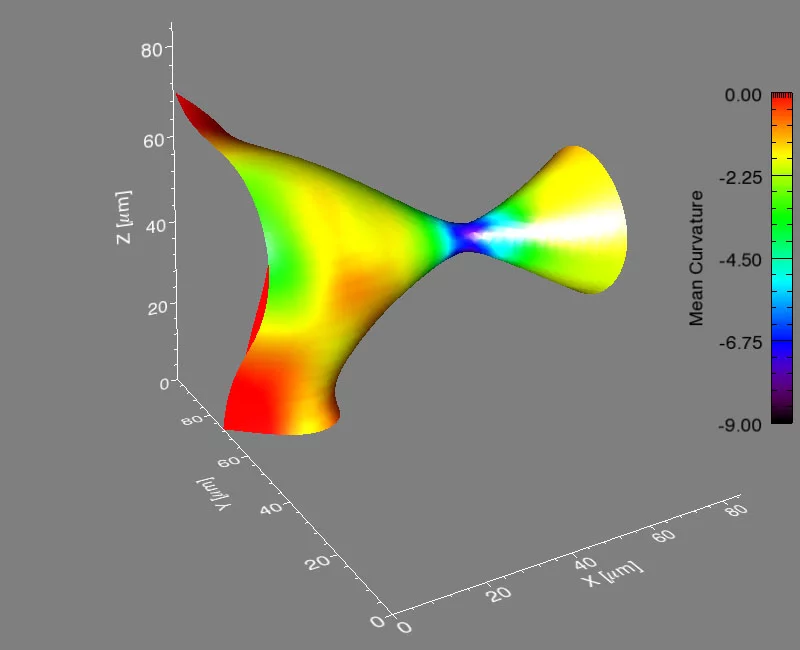
It’s a problem that materials scientists have considered for years: how does a material composed of more than one phase evolve when heated to a temperature that will allow atoms to move? A phase is a region of a material that has a unique composition or atomic structure. For instance, ice (crystalline) and water (liquid) are two different phases. In many cases, a rod-like phase embedded in another will break up into smaller domains, which results in significant changes in the properties of the material. For example, imagine that a stream of water coming out of a faucet is the rod-like phase and it subsequently breaks up into droplets when, for example, flow pressure is decreased or distance from the faucet is increased.
Now, researchers at Northwestern University in the USA, together with collaborators from Risø National Laboratory for Sustainable Energy in Denmark and the Swiss Light Source at the Paul Scherrer Institut in Switzerland have answered two important questions about this break-up process: How does it happen, and how long does it take?
Researchers found the answer is universal among materials — a rare case in the materials world — and their results were published online on August 1 in the journal Nature Physics.
The international group, led by Peter Voorhees (Northwestern University), Erik Lauridsen (Risø National Laboratory), and one graduate student from each institution, spent five, 24-hour days at the TOMCAT beamline of the Swiss Light Source (SLS) at the Paul Scherrer Institut, working with Marco Stampanoni and Federica Marone (PSI) using synchrotron-based X-ray tomographic microscopy (in a relatively new approach that allows for very fast measurements of a material in three-dimensions and in time). They investigated the evolution of the rod-shaped phases during the break-up process in a specialized high-temperature setup. They collected data in real time—observing the interfaces of the rods as they broke up. After five days, they had over two terabytes of real-time information to analyze.
They found that the shape of the interfaces during break-up becomes universal, no matter what material is used. This sort of universality allows them to predict the dynamics of the break-up process in a vast array of materials, like steels, aluminum alloys, and even noncrystalline materials like polymers.
Voorhees then took the experimental data to Michael Miksis (Northwestern University), and the two of them and another graduate student described the process theoretically. They developed equations to calculate the time required for the pinching process to happen, and they found that the kinetics of the process are fixed early on and are the same, no matter the material.
If it is a rod, and it is pinching off by diffusion in the material, then the universal kinetics we observed will describe it,
Voorhees said.
The process has an impact on a wide range of materials, including steel and polymers. For example, many metal parts are made by a casting process, where a liquid metal is poured into a mold and solidifies into the shape of the part. As the liquid solidifies it forms tree-shaped structures called dendrites, and if one of the arms of the dendrites breaks off, it can lead to a change in the properties of the solidified material. The airplane industry, for example, spent a significant amount of time developing solidification methods to avoid this problem when casting jet turbine blades. Another example is polymer solar cells, which use a complicated mixture of two types of polymers. When heated up, the mixture evolves by a process that involves pinching, which ultimately alters the properties of the mixture and the efficiency of the solar cell.
The experiments took place two and a half years ago, and Voorhees and his colleagues are still analyzing the data and their initial results.
This work is truly a collaborative effort,
Julie Fife (previous graduate student under Voorhees and now at PSI) said. We have international expertise in all avenues, and combining this with the novel facilities available at the TOMCAT beamline, we created a winning team. To have achieved results that are universal and thus applicable to many material systems was one the main goals of this project, and its success is a direct result of this collaboration.
The title of the paper is, Universality and self-similarity in pinch-off of rods by bulk diffusion.
Text: Emily Ayshford, Northwestern University
About PSI
The Paul Scherrer Institute develops, builds and operates large-scale, complex research facilities, and makes these facilities available to the national and international research community. The Institute’s own research focuses on solid-state physics and the materials sciences, elementary particle physics, biology and medicine, as well as research involving energy and the environment. With a workforce of 1300 and an annual budget of about 260 million CHF, PSI is the largest research institution in Switzerland.
Contact
Prof. Peter W. Voorhees, Department of Materials Science and Engineering,Northwestern University, Evanston, Illinois, USA,
Phone: +1 847 491 7815, e-mail: p-voorhees@northwestern.edu
Dr. Julie Fife, Laboratory for Macromolecules and Bioimaging, Department for Synchrotron Radiation and Nanotechnology,
Paul Scherrer Institute, 5232 Villigen PSI, Switzerland.
Phone: +41 56 310 5840, e-mail: julie.fife@psi.ch
Prof. Marco Stampanoni,
Institute of Biomedical Technology at the ETH Zürich and Laboratory for Macromolecules and Bioimaging at the Paul Scherrer Institute,
5232 Villigen PSI;
Phone: +41 56 310 4724; e-mail: marco.stampanoni@psi.ch
Original Publication
Universality and self-similarity in pinch-off of rods by bulk diffusionLarry K. Aagesen, Anthony E. Johnson, Julie L. Fife, Peter W. Voorhees, Michael J. Miksis, Stefan O. Poulsen, Erik M. Lauridsen, Federica Marone & Marco Stampanoni, Nature Physics,
Advance Online publication, 1 August 2010,
DOI: 10.1038/nphys1737