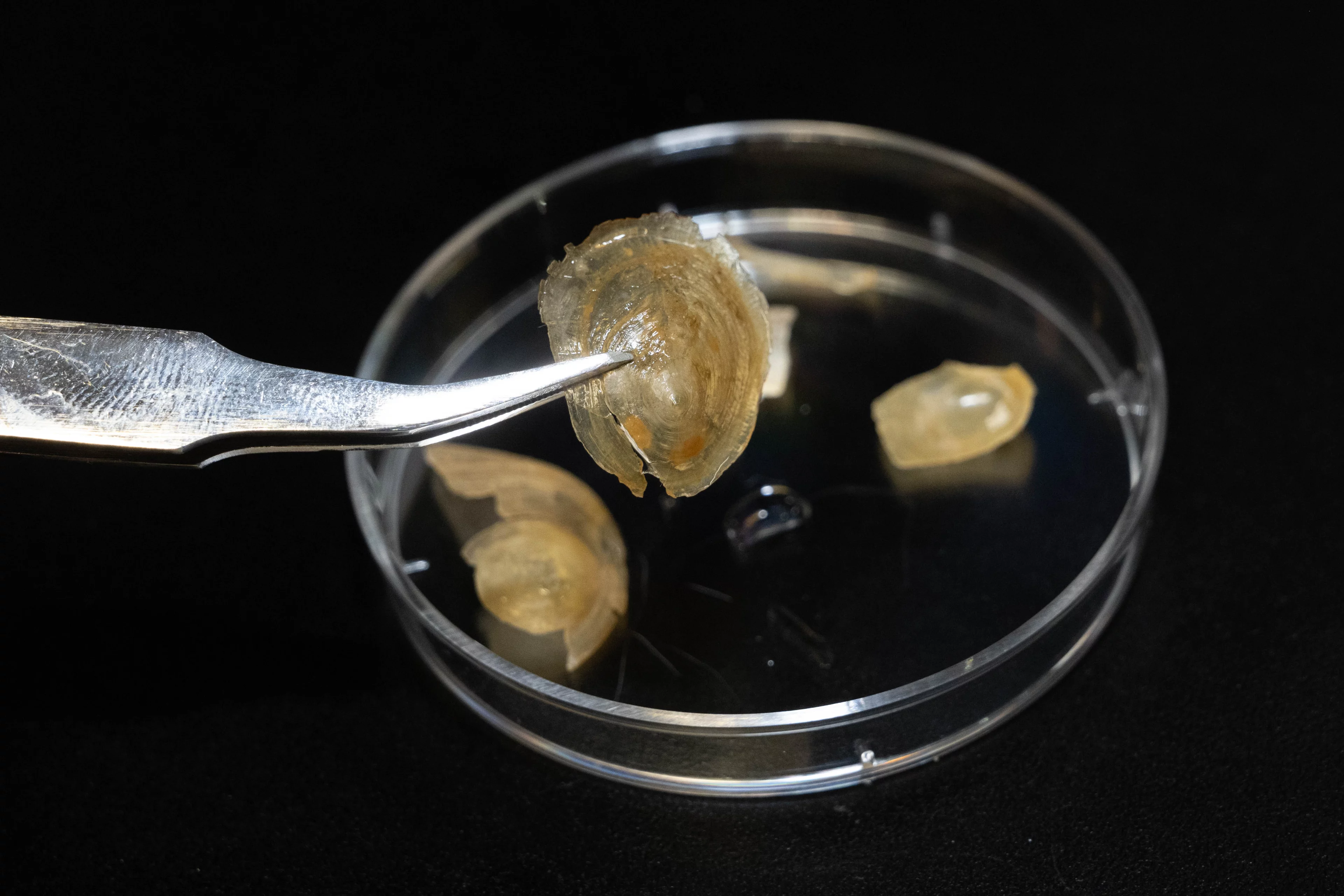
An international research team with participation of the Paul Scherrer Institute PSI has revealed a secret about a marine animal's shell: The researchers have deciphered why the protective cover of the brachiopod Discinisca tenuis becomes extremely soft in water and gets hard again in the air. The study appears today in the journal Nature Communications.
The brachiopod Discinisca tenuis lives on the west coast of Africa. It has a mineral-rich shell that protects it from harmful environmental influences. Bathing the shell in water leads to a structural change in the material: The flat, hard shell becomes so flexible that it can even be folded up without breaking. With the help of the Swiss Light Source SLS, the researchers have deciphered exactly how this transformation takes place.
The phenomenon was discovered by chance a few years ago by Fabio Nudelman, a materials chemist currently at the School of Chemistry, University of Edinburgh in Scotland. Maggie Cusack, who was recently appointed president of Munster Technological University in Ireland, had provided Nudelman with shells of the brachiopod Discinisca tenuis, which originally came from Namibia. When he wanted to wash the hard object, it suddenly became soft and flexible in contact with water. The shell had absorbed liquid and thereby changed its structure. The process was reversible: When the shell dried, it became hard and brittle again.
Together with colleagues from six countries, Nudelman set out to discover what exactly takes place during this unexpected transformation. "In its composition, the shell resembles bone," he explains. "But bone doesn't change its structure when it gets wet." The same goes for clams: If the animals need to adapt the properties of their shell to different environmental conditions, they normally have to rework the material in a lengthy and energetically costly process, by resorbing and redistributing minerals. It doesn't work simply through the absorption of water.
Hybrid material with a special trick
It was so-called cryo-tomography, performed at the Swiss Light Source SLS, that "opened the door to reveal the secret," says Johannes Ihli, a PSI researcher at SLS. With this technique, the researchers examined the material as if under a very high-resolution microscope, and in fact at extremely low temperatures. "At room temperature it would not have been possible, since the high-energy X-ray light would immediately alter the sensitive shell structure," Ihli explains.
The brachiopod's shell, which is no more than half a millimetre thick, consists of a hybrid material: mainly inorganic mineral in which organic polymers made from proteins and sugars are embedded. Bones, clam shells, and teeth are structured in a similar way way out of a mixture of organic and inorganic material. The mineral that constitutes the main component of the shell is a type of fluoroapatite – similar to the material that makes up the enamel of our teeth.
Tiny nanocrystals of this material are arranged in layers. Nudelman compares it to brick walls: "In this analogy, the bricks are the nanocrystals, and the mortar between the bricks consists of organic molecules such as chitin and proteins." As the researchers observed, this "mortar" can absorb large amounts of water, causing it to swell up. Through the storage of water, it changes its structure: It becomes soft, and the bricks become movable with respect to each other. "Then water acts like a lubricant between the individual nanocrystals," Ihli explains. "The crystals can then slip against each other." Through this movement, the shell becomes flexible. The researchers found a network of pores in the shell that was especially effective in guiding water inside and rapidly distributing it throughout the material.
Evolutionary advantage
Discinisca tenuis lives in large clusters in tidal zones on the coast where, depending on the tide, the animals are exposed to strong waves or calm waters. The researchers speculate that it is probably advantageous if the animals can quickly adapt the softness or hardness of their shell to the respective situation: "This could prevent damage to the shell and thus be a key to the animals' survival," they write in the study. The phenomenon may even be more widespread than suspected: "We don't know how many other animal species there might be that have this kind of property," says Nudelman.
Aside from biology and evolution, the newly gained insights are also of interest for materials science: The development of a hard, brittle material whose stiffness can be controlled could hold promise for many applications. Sports clothing or helmets, for example, might be able to flexibly adapt to movements and always offer the protection required depending on the impact. Harnessing this phenomenon could also prove useful in developing bone-replacement materials.
Participating research institutions
- Paul Scherrer Institute PSI, Villigen, Switzerland
- University of Bayreuth and Bavarian Polymer Institute, Bayreuth, Germany
- ETH Zurich and University of Zurich, Switzerland
- University of Bologna, Italy
- University of Edinburgh, UK
- Heriot-Watt University, Riccarton, UK
- University of Cambridge, UK
- Center for Functional Nanomaterials and National Synchrotron Light Source II, Brookhaven National Laboratory, Upton, New York, USA
- Munster Technological University, Cork und Kerry, Ireland
Contact
Original publication
-
Ihli J, Schenk AS, Rosenfeldt S, Wakonig K, Holler M, Falini G, et al.
Mechanical adaptation of brachiopod shells via hydration-induced structural changes
Nature Communications. 2021; 12(1): 5383 (12 pp.). https://doi.org/10.1038/s41467-021-25613-4
DORA PSI
About PSI
The Paul Scherrer Institute PSI develops, builds and operates large, complex research facilities and makes them available to the national and international research community. The institute's own key research priorities are in the fields of future technologies, energy and climate, health innovation and fundamentals of nature. PSI is committed to the training of future generations. Therefore about one quarter of our staff are post-docs, post-graduates or apprentices. Altogether PSI employs 2300 people, thus being the largest research institute in Switzerland. The annual budget amounts to approximately CHF 460 million. PSI is part of the ETH Domain, with the other members being the two Swiss Federal Institutes of Technology, ETH Zurich and EPFL Lausanne, as well as Eawag (Swiss Federal Institute of Aquatic Science and Technology), Empa (Swiss Federal Laboratories for Materials Science and Technology) and WSL (Swiss Federal Institute for Forest, Snow and Landscape Research). (Last updated in June 2024)