The molecule dicarbon (C2) is present in all carbon-containing flames. (C2) is the origin of the green-blue colour inside a candle flame and might play a key role in the formation of soot. Now, for the first time, scientists from the Paul Scherrer Institute have rendered a previously invisible (C2) energy state, a so-called dark state, visible. Not only is its discovery interesting for combustion researchers; it also solves a century-old puzzle in the spectrum of this omnipresent molecule.
If you want to characterise a molecule, you have to analyse its spectrum, a molecule’s fingerprint. It contains information on which energy states the molecule can even assume in the first place. And, in turn, these energy states largely determine how the molecule behaves chemically and physically.
A team of PSI researchers have now discovered an energy state in the C2 molecule that has eluded scientists for over a century. It is as if a new, previously unseen line were to be found in a human fingerprint. The discovery is expected to help shed light on the role of C2 in processes where high temperatures and pressure prevail.
A puzzle in flames, stars and comets
Not only is the C2 molecule of great relevance in combustion research, where it serves as a marker for a flame’s spread; astrophysicists also want to understand it better as it is found in interstellar dust, which darkens the light from stars. C2 is also found in the spectrum of the sun and other stars. In certain stars, the C2 spectrum is so intense that they are dubbed “carbon stars”. Moreover, back in around 1910 C2 was already thought to be one of the molecules that characterises part of the spectrum of comet tails. The problem until now was that certain peaks occurring in these spectra could not be ascribed to any known state of the molecule. The discovery of the as yet unverified C2 energy state by PSI researchers has therefore caused much attention in several research fields.
Evidence thanks to spot landing
The existence of the C2 energy state now detected has actually been suspected for a number of years. However, it was only the unique, precision work of the researchers involved that provided definitive proof.
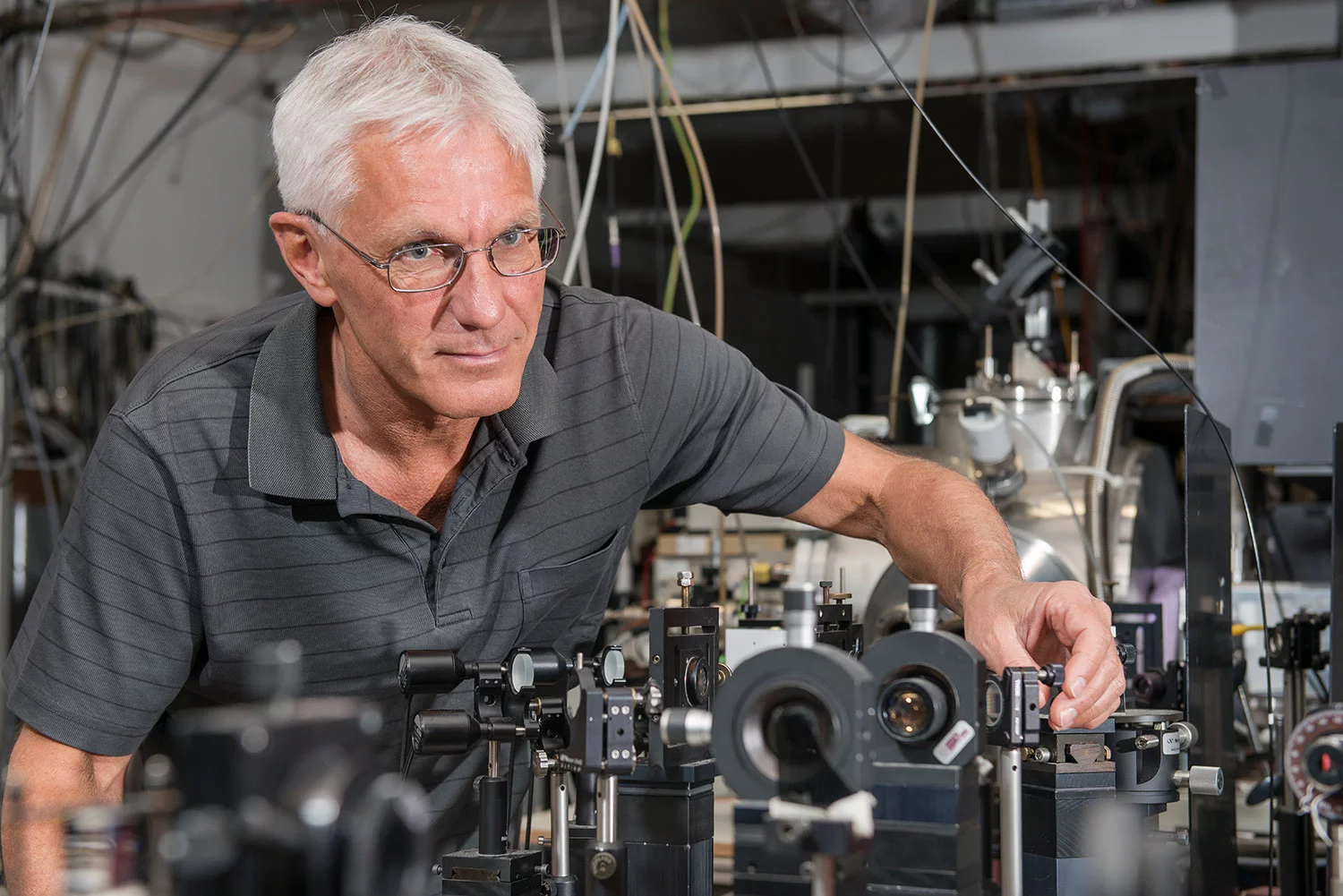
First of all, C2 needed to be produced in an environment where the otherwise short-lived molecule could survive long enough for the measurements to be made. With this in mind, the PSI researchers generated a molecular beam comprising a substantial abundance of C2 molecules by exposing a mixture of acetylene and the noble gas argon to an electrical discharge. The C2 formed from the discharge as gas, which was then expanded through a thin slit into a vacuum chamber at supersonic speed. The established molecular beam isolates the C2 molecules sufficiently not to destroy each other in collisions.
The PSI researchers then used an elaborate and ultra-precise laser technique to measure the C2 spectrum and identify the new energy state. The details of the method, which is referred to as two-colour resonant four-wave mixing, are complex but it basically works as follows: you shine three intersecting laser beams, each with extremely precisely defined wavelengths, onto the C2 molecules, which induces the molecules to pass into a precisely determined state with a higher energy level. When the excited molecules calm down again, they emit a laser beam, which the researchers can measure. The wavelength of the laser beam transmitted reveals how much energy the molecules absorbed during the excitation, which in turn provides an indication of the energy of the state sought.
The technique is very sensitive and precise: you only receive a signal if you bring the molecules to the very state sought with the lasers, the wavelengths of which are very precisely defined – you have to hit the wavelength of the lasers in a kind of spot landing. The detection is a lot easier, however: compared to other laser-spectroscopic techniques, the transmitted signal light to be measured is not emitted randomly but in a very specific direction, which is determined by the way in which the three laser beams intersect. In other words, you only have to place your detector in this one direction to register practically the entire signal at full strength.
A dark state reveals itself
The analysis of the readings and highly accurate calculations revealed that the new state discovered is a so-called dark state. This means that the molecules are unable to reach this state directly by absorbing energy, such as from a laser, as transitions to this state are essentially impossible through fundamental natural laws. Using the novel method, however, the PSI researchers were able to isolate so-called gateway states, which provided exact information on the dark state. This enabled them to prove indirectly that certain peaks in the spectrum could be traced back to this “dark state”.
PSI scientist Peter Radi is convinced that the new-found “line” in C2’s fingerprint will help clarify many details in combustion processes. C2 forms at the flame front, i.e. precisely where the fuel and air mixture ignites. A better understanding of the C2 spectrum makes it easier to detect and thus illustrate a flame’s spread. Moreover, the new-found C2 state might play a key role in the formation of soot. After all, the C2 molecules can achieve the new state through collisions, in which they are incorporated into larger carbon chains that are typical of soot. And then this century-old, repeatedly observed puzzle in the spectrum of C2 will have been solved once and for all.
You could barely hope for any more enlightenment from a “dark” state.
Text: Paul Scherrer Institute/Leonid Leiva
Additional information
Combustion Research LaboratoryContact
Dr. Peter Radi, Combustion Research Laboratory, Paul Scherrer Institute,Telephone: +41 56 310 41 27, E-mail: peter.radi@psi.ch