An ingenious measurement chamber
Natural-gas vehicles are on the way, and they need catalytic converters for the exhaust too. So far, catalytic converters for gasoline-powered vehicles have been modified to fill this gap; however, these are not geared towards removing the climate-harmful methane that the exhaust from natural-gas vehicles contains. While work is under way at Empa, the Swiss Federal Laboratories for Materials Testing and Research, to optimise natural-gas engines and catalytic converters, the PSI is specialising in the development of research methods that enable very precise observation of processes in catalytic converters. For different methods, different types of radiation are used, which yield different insights into the catalytic converters. Until now it was necessary to set up the catalytic converter to be studied in a new measurement chamber for each of these methods. Now researchers at the PSI have developed a universal measurement chamber in which catalytic converters can be studied with a variety of different methods, yet always under the same conditions.
Vehicles powered by natural gas are on the way. The advantage is obvious: Among all of the hydrocarbon-based fuels, natural gas has the lowest CO2 emission per unit of energy, because it is composed of the energy carrier methane (CH4). When it comes to synthetic natural gas from biomass, the advantage is even greater since the carbon content derives from a renewable source.
However, the market for natural-gas vehicles is still small, so that it hardly pays off for the automobile manufacturers to develop their own finely tuned catalytic converters for the exhaust from natural-gas engines. Instead, they prefer to adapt catalytic converters for petrol engines, which are already on the market. The problem there is that the emissions from gas engines contain small amounts of methane that should, as far as possible, be prevented from getting into the atmosphere since methane contribution to the greenhouse effect is large.
While work is under way at Empa, the Swiss Federal Laboratories for Materials Testing and Research, to technically optimise gas engines and the catalytic converters they require, the PSI is specialising in ingenious methods with which the processes in catalytic converters can be observed very precisely. Improved materials for catalytic converters can be developed following these activities, together with ideas for more efficient operation.
An effective coating
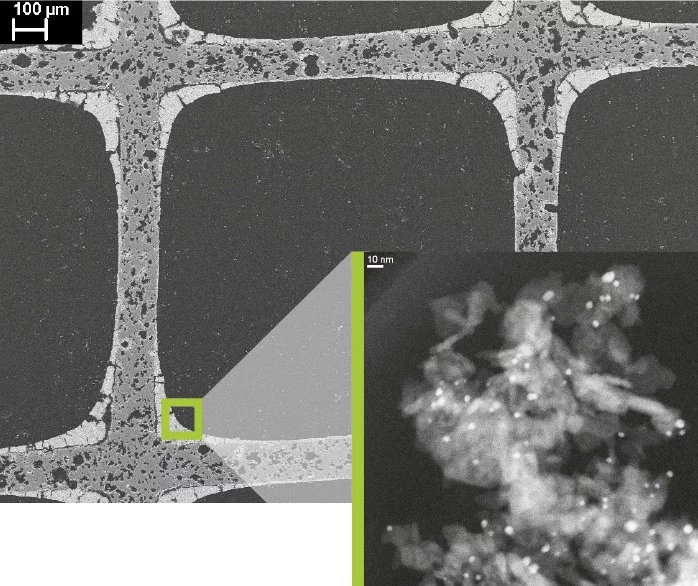
In automotive catalytic converters, the exhaust is guided through a large number of parallel ceramic channels. Their surface is coated with a powder material, the actual catalyst, which consists of particles of noble metals, often palladium among other components. The pollutants in the exhaust react with this coating and are converted into water, nitrogen, and CO2. Methane molecules are not so easy to break down with these common catalysts, though, since other constituents of the exhaust bind more readily than methane to the catalyst, and thus occupy the needed catalyst surface area.
Davide Ferri, senior scientist in the Catalysis for Energy research group at the Paul Scherrer Institute, elaborates: To compensate for that, in catalytic converters for gas engines the industry applies a somewhat thicker coating of palladium and counts on that being sufficient to break it all down. But our goal is the optimisation of the coating to increase efficiency and economise on precious material.
To that end, the research group must study the behaviour of the noble-metal particles and see how they react with the pollutants. In addition, the group tries to trace whether the particles aggregate with time and under different conditions in such a way to cause a loss of performance of the catalytic converter over time.
Ferri wants to know what initiates such ageing processes, how they proceed, and how they can be delayed. We can trace such processes in detail, because through a clever combination of several measurement methods we can track how the atoms of palladium and the molecules of the pollutants react with each other. Our specialty is to actually observe the catalytic converter in the laboratory under realistic conditions such as fluctuations in temperature and oxygen content,
he reports. For each measurement method, the researchers use precisely matched radiation that can only penetrate certain materials.
Normally every observation requires another specialised measurement chamber in which the reaction conditions can be simulated. The catalyst must be mounted in each dedicated chamber, a time-consuming process. A better solution has been developed at the PSI: We now have a measurement chamber, where one only needs to switch the window to fit the type of radiation.
Thus several measurements can be carried out, not only in significantly less time, but also under absolutely identical conditions in one and the same chamber.
Now the researchers are able to search even more efficiently for better catalysts. They will not rest until they have found a catalyst that will convert every last methane molecule.
Text: Alexandra von Ascheraden
Contact
Dr. Davide Ferri, Catalysis for Energy, Paul Scherrer Institute, 5232 Villigen PSI, SwitzerlandTelephone: +41 56 310 27 81, e-mail: davide.ferri@psi.ch