Forest trees use carbon not only for themselves; they also trade large quantities of it with their neighbours. The extensive carbon trade among trees – even among different species – is conducted via symbiotic fungi in the soil.
It is well known that plants take up carbon dioxide from the air by photosynthesis. The resulting sugar is used to build cellulose, wood pulp (lignin), protein and lipid – the building blocks of plants. While growing, the tree transports sugar from its leaves to the building sites: to the branches, stems, roots and to their symbiotic fungi below ground (mycorrhizal fungi).
Carbon dioxide shower for trees
Dr. Tamir Klein and Prof. Christian Körner of the University of Basel report, that this sugar export goes further than previously thought. In a forest near Basel the researchers used a construction crane and a network of fine tubes to flood the crowns of 120 year old and 40 meter tall spruce trees with carbon dioxide that carried a label. The researchers used carbon dioxide that had a certain recognizable feature.
For that, the researchers exploit the fact that the carbon in carbon dioxide comes in two different versions, or isotopes: the common 12C and the very rare, somewhat heavier 13C. In the carbon dioxide that the researchers exposed the trees to, the proportion of 13C was smaller than what occurs naturally in the air. The specific proportions of carbon isotopes both in the air and in the gas that the researchers provided were continuously analysed by Dr. Rolf Siegwolf, leader of the Ecosystem Fluxes Group at the Paul Scherrer Institute. For the trees, it makes no difference which carbon they get. The researchers, though, can measure the ratios of the two isotopes at various parts in the trees and thus establish where the carbon taken up through photosynthesis gets transported to, from the treetops to the tips of the roots.
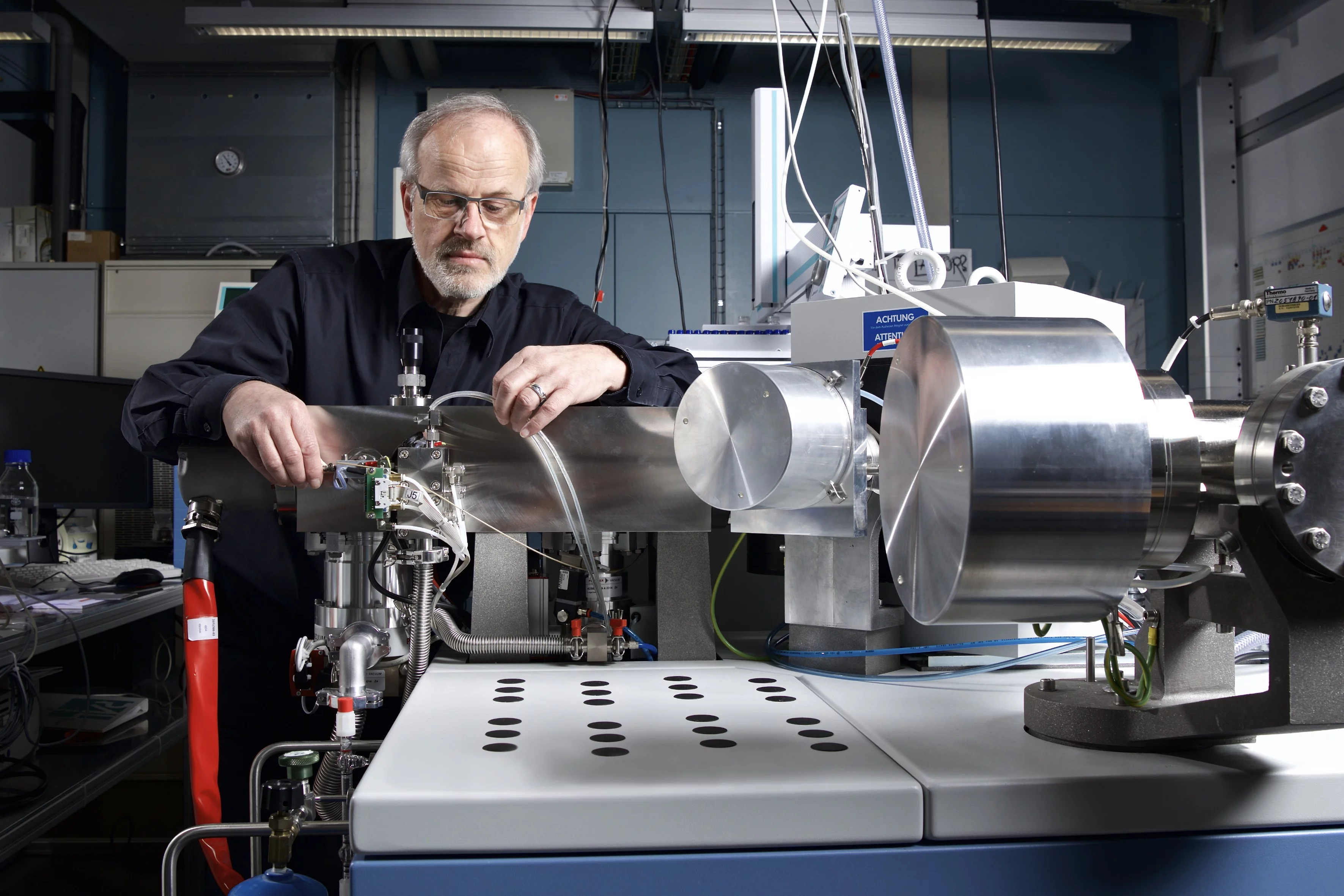
As a method of analysation, the researchers use a so-called atomic mass spectrometer that is operated by Siegwolf’s research group. For this study they took samples, at different times and from various parts of the trees and as well as other plants in their immediate surroundings, and subsequently determined the ratio of 12C and 13C concentrations in the lab. In this way the researchers found that the labelled carbon showed up not only in new roots of the treated spruce trees: The same signal was also found in the roots of neighbouring trees that had not been given labelled carbon dioxide, even in trees of different species.
Forest is more than the sum of its trees
The only way the carbon could have been exchanged from spruce to beech, pine or larch tree – or vice versa – is by the network of tiny fungal filaments of the shared mycorrhizal fungi. Understory plants which partner up with other types of fungi remained entirely unmarked. The research group called the discovered exchange of large quantities of carbon among completely unrelated tree species in a natural forest a big surprise
.
According to the researchers, the discovery questions the concept of tree individuality with regard to the single largest constituent of the biosphere, tree carbon. Furthermore, the results of the study funded by the Swiss National Science Foundation add a new dimension to the role of mycorrhizal fungi in forests. Evidently the forest is more than the sum of its trees
, comments Prof. Christian Körner the findings.
Text: Based on a news release from the University of Basel, with additions from the Paul Scherrer Institute
Background: Concentration ratios of stable isotopes
Many chemical elements that occur in great abundance in our environment — amongst others the elements oxygen (chemical symbol O) and carbon (C) — come in two stable versions, that is, stable isotopes whose atoms have slightly different masses. By determining the proportions of the isotopes of an element in plants, researchers can investigate interactions between the environment (climate, geography, anthropogenic influences) and vegetation. This type of study is a specialty of the Ecosystem Fluxes Group at the Paul Scherrer Institute PSI.
Central to these studies are the effects of the changing climate (higher temperatures and altered hydrological balance), increasing concentration of CO2, and the effects on vegetation of contaminants in the air (ozone, oxides of nitrogen, SO2). During the plants’ metabolic processes, the lighter 12C isotopes are taken up more readily than the heavier ones, in contrast to the oxygen, where the heavier 18O isotopes are preferredly incorporated. Changes in isotope ratios such as 13C/12C are highly specific metabolically, and thus they allow inferences to be made about how plants are affected by environmental conditions — drought, pollution, temperature changes, or variations in light exposure, for example.
As the collaboration with the University of Basel shows, the study of concentration ratios of stable isotopes is very well suited for tracking material transport in plants, or even in entire ecosystems. The research findings described above would not have been possible without the analysis of stable isotopes.
Concentration ratios can be determined very precisely with isotopic mass spectrometers. The PSI has five mass spectrometers at its disposal, as well as the facilities needed to prepare samples (extracted parts of plants, for example) for analysis.
Additional information
Ecosystem Fluxes Group at the Paul Scherrer Institute
The Swiss Canopy Crane Project (SCC)
Contact
Dr. Rolf Siegwolf, Ecosystem Fluxes GroupPaul Scherrer Institute, 5232 Villigen PSI, Switzerland
Telephone: +41 56 310 27 86, e-mail: rolf.siegwolf@psi.ch
Prof. Dr. Christian Körner, University of Basel, Department of Environmental Sciences
Telephone: +41 61 267 35 10, e-mail ch.koerner@unibas.ch
Original Publication
Belowground carbon trade among tall trees in a temperate forestT. Klein, R. T. W. Siegwolf, C. Körner
Science 15. April 2016: Vol. 352, no. 6283, pp. 342–344
DOI: 10.1126/science.aad6188