Experiments at SwissFEL will help us understand in detail how one substance is transformed into another during a chemical reaction. Highest priority will be given to catalytic reactions, as these have numerous industrial applications. This research will point the way towards more energy-efficient industrial processes and environmentally-friendly energy carriers.
In nature, substances are transformed into others all of the time - one substance disintegrates, and a new one is formed from its constituents. The chemical industry has produced countless substances that are now key parts of our everyday lives - fuels, plastics, medicines or fertilisers - using chemical conversions. Yet even with manufacturing processes that have been used for decades, the reactions involved are still not understood in detail. In practical terms, this means the efficiency of the reactions used may be sub-optimal – that is, the energy consumed may be unnecessarily high, only part of the basic material may be consumed, and more waste could be produced than is necessary.
Too small and too fast for current techniques
The processes occurring in chemical reactions are incredibly fast. Whereas the initial substances may be converted into end products on the millisecond scale (thousandth of a second), the motions of the individual molecules and atoms involved take only a few femtoseconds (a femtosecond is a millionth of a millionth of a thousandth of a second). And even though some existing experimental tricks have enabled scientists to elucidate the various atom combinations formed during some reactions, the pathways from one intermediate step to the next remain obscure. Thanks to SwissFEL – and similar facilities – however, this is about to change. The light pulses it generates are so short that parts of a molecule only move a short distance whilst they are being illuminated. And because these pulses are composed of X-rays, the position of individual atoms can be visualised. Thus, the particles involved in the reaction can be “photographed” whilst they are in motion – similar to photographing an athlete using a camera with a short exposure time.
Improving familiar processes, developing new processes
Catalytic reactions will be prioritised for studies at SwissFEL. These play an important role in the chemical industry – around 80% of all chemical products are manufactured using such reactions. It therefore pays to understand them in greater detail, since making them more efficient could result in huge reductions in energy consumption. A classic example of an industrially important catalytic reaction is the synthesis of ammonia, which forms the basic material for the production of artificial nitrogenous fertilizers.
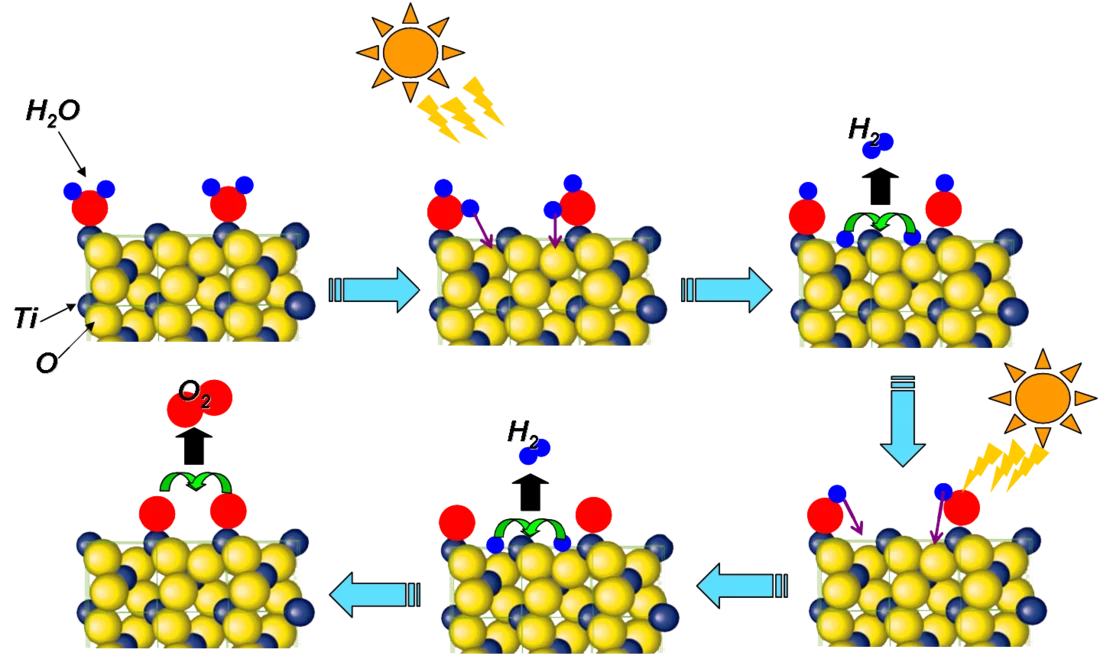
Research at SwissFEL will allow the development of novel processes based on catalytic reactions. A key process in this context is "artificial photosynthesis". Such a process would allow methane or methanol to be produced from carbon dioxide and water using solar energy. These products could be then used as the raw materials for further chemical products and fuels. An artificial photosynthetic process would allow new fuels to be produced from the carbon dioxide released during combustion, and these fuels could be used without increasing the amount of CO2 in the atmosphere. At present, corresponding processes are too inefficient to be scaled-up for industrial applications, but in the long term, experiments at SwissFEL could point the way towards improving their efficiency.
Converting substances using supports – what are catalytic reactions?
Catalytic reactions rely on catalysts. These are not used up during a reaction, but are essential if some reactions are to take place efficiently. In some cases, when one substance is being converted into another, a large amount of energy is needed to break the bonds between individual atoms in a particular molecule. However, if the molecule is attached to a catalyst first– often the surface of nanometre-sized metallic particles – the bond holding them within the molecule can be significantly weakened, which allows them to be broken up using less energy. The efficiency obtained is strongly dependent on the detailed structure of the individual catalysts used. Studies at SwissFEL will help us understand how a catalyst should be structured to allow specific reactions to be optimised.