Researchers at the Paul Scherrer Institute are studying several classes of proteins with the aim of understanding their structure and function. Research topics include the study of proteins, which, as components of the cytoskeleton of a cell, give the cell its shape and enable its movements; the study of membrane proteins that determine which substances must be transported into or out of a cell and how signals are transferred into a cell. Apart from their own research on protein structures, researchers are also involved in the development of new techniques for determining protein structures – in particular with synchrotron light at the Swiss Light Source (SLS) – and for the automated production of the large quantities of proteins required.
Many of the results achieved could contribute to the development of new cancer treatments in the long term, since research at PSI helps towards a better understand of the vital processes in biological cells. This understanding could contribute to the targeted blocking of such processes in cancerous cells and to the slowing down of their further growth, or to causing their destruction.
Proteins – the basic building blocks of life
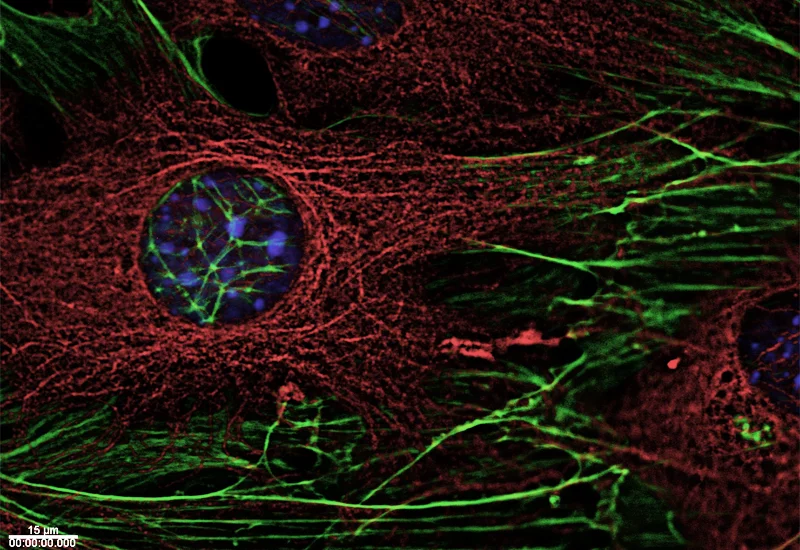
Proteins form the basic building blocks of living organisms. These complex molecular structures are responsible for innumerable tasks in living cells – they give cells their shape, transport substances through cells, or receive signals which reach cells from outside and guide them through the cell membrane into its interior. The exact function of a protein is directly connected in this way to its structure – not only to the sequence of its fundamental building blocks (amino acids), but particularly to its three-dimensional shape.Proteins in the cytoskeleton
Three types of polymer filaments are present in living cells that jointly make up the cytoskeleton, which stabilises cells and maintains their shape. However, they do not form a stiff framework, but react flexibly to external influences and the specific needs of the cell, and are actively involved in numerous vital processes in the cell. As needed, its filaments enable a cell to change its specific shape and to move. During cell division, they separate the chromosomes destined for both newly forming cells, or participate in transporting cellular material to its destination within the cells.
Researchers at PSI are studying the structure of proteins involved in the cytoskeleton in order to understand the relationship between structure and function. Therefore, the scientists are engaged in a study of the proteins which connect the ends of cytoskeleton filaments with various different cell components, such as chromosomes or the cell membrane. As a result, they have been able to explain the mechanism which enables these proteins to recognise the filament ends and bind themselves to them.
Communication through the cell membrane
As part of a living organism, a living cell is in constant dialogue with its surroundings. Thus, signals reach the cell from outside which trigger processes within the cell. At the same time, the exchange of substances between the cell interior and its surroundings has to be managed. All these processes are controlled by special proteins which can change their shape, and thereby their behaviour, depending on the external conditions.
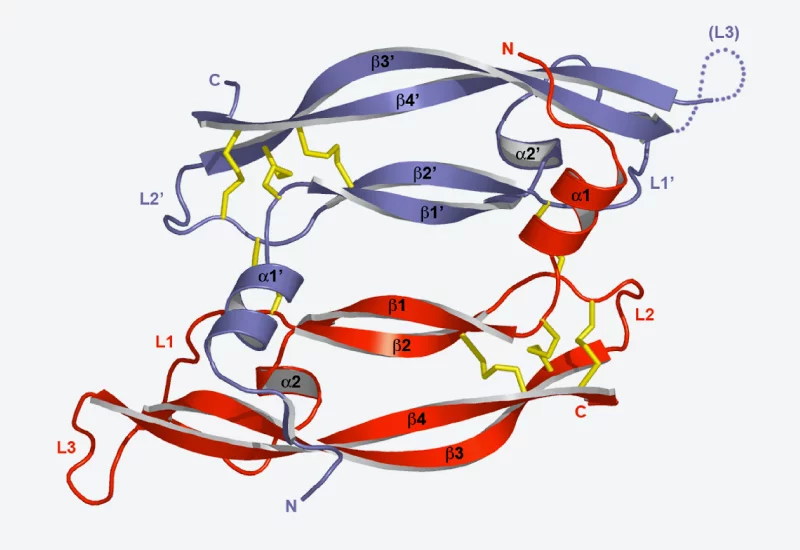
Hence there are a great number of proteins located in the membrane of a cell which pass signals from the exterior to the interior. A signal of this kind can be, for example, from a neurotransmitter molecule transmitting a signal from one nerve cell to the next, or a molecule (denoted by the abbreviation VEGF) which stimulates certain cells to develop into blood vessels. VEGF binds to the ends of a protein, a so-called receptor, which can be found on the outside of the cell. The binding of VEGF to the receptor causes a change in the receptor structure. Within seconds, this structural change also has an effect on the inner surface of the cell membrane and triggers certain chemical reactions within the cell, which lead to its transformation.
Other proteins control the exchange of substances with the surroundings of the cell. For example, signal transmission in a nerve cell is based on the fact that certain ions flow into or out of the cell at the right moment. For this purpose, certain membrane proteins act as ion channels, which transport the ions through the membrane.
The aim of researchers at PSI is to determine the structure of various membrane proteins, from which they are able to derive their exact function. In this way, they are ascertaining, for example, how the structures of proteins change when they are connected to a signal molecule.
New methods in structural biology
For their work, researchers use the entire breadth of structural biology methods, but are also concurrently developing new methods. These range from automated processes for the production of the large quantities of proteins required, to exact experimental methods using synchrotron light at the SLS.
The quantities of proteins required for the studies of structures by far exceed the quantities in which they occur in living organisms. Researchers at PSI are therefore working on the development of methods in which complex production processes are automated with the aid of robots
.
With the Swiss Light Source, PSI has at its disposal a facility which opens up unique possibilities for structural biology. For example, researchers are working on adapting small-angle scattering with synchrotron light to suit the needs of structural biology. This method allows the determination of the external three-dimensional shape of proteins which are dissolved in a fluid. The method supplements the well-established protein crystallography with synchrotron light, which currently supplies the best protein structures, provided the proteins are present as crystals. However, proteins frequently cannot be crystallised, and, in other cases, the fact that the proteins are assembled to form crystals has an influence on their shape. The small-angle scattering method ideally supplements the common methods used today for protein structure determination.
Additional Information
- Research using synchrotron radiation
An important tool for structural biology - Swiss Light Source SLS
PSI's synchrotron light source - Laboratory of Biomolecular Research
Web page of the Laboratory