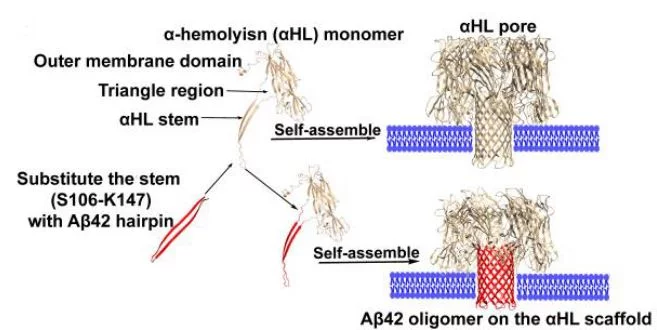
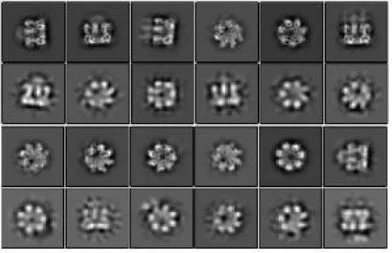
A functional and structural Aβ42-oligomer equivalent, created by fusing Aβ42 to the oligomerizing, soluble domain of the α-hemolysin (αHL) toxin, share major structural, functional and biological properties with Alzheimer’s wild-type Aβ42 oligomers. Cryo-EM studies reveal that the αHL-displayed oligomers structurally remain a well-defined stoichiometry, providing a stable mimetic antigen for the development of conformation-specific antibodies against Alzheimer’s Disease.
Amyloid-β peptide (Aβ) oligomers are pathogenic species of amyloid aggregates in Alzheimer’s disease. Like certain protein toxins, Aβ oligomers permeabilize cellular membranes, presumably through a pore formation mechanism. Due to their structural and stoichiometric heterogeneity, the structure of these pores remains to be characterized. We studied a functional Aβ42 pore equivalent, created by fusing Aβ42 to the oligomerizing, soluble domain of the α-hemolysin (αHL) toxin. Our data reveal Aβ42-αHL oligomers to share major structural, functional and biological properties with wild-type Aβ42-pores. Single-particle cryo-EM analysis of Aβ42-αHL oligomers (with an overall resolution of 3.3 Å) reveals the Aβ42-pore region to be intrinsically flexible. We anticipate that the Aβ42-αHL oligomers will allow studying many of the features of the wild type amyloid oligomers that cannot be studied otherwise, and may represent a highly specific antigen for the development of immuno-base diagnostics and therapies.
Contact
Dr. Jinghui Luo
Laboratory of Nanoscale Biology
Paul Scherrer Institut
Forschungsstrasse 111, 5232 Villigen PSI, Switzerland
Telephone: +41 56 310 4764
E-mail: jinghui.luo@psi.ch
Original Publication
Cryo-electron microscopy imaging of Alzheimer’s amyloid-beta 42 oligomer displayed on a functionally and structurally relevant scaffold
Jinming Wu, Thorsten B. Blum, Daniel P Farrell, Frank DiMaio, Jan Pieter Abrahams, Jinghui Luo
Angew. Chem. Int. Ed. 10.1002/anie.202104497
DOI: 10.1002/anie.202104497