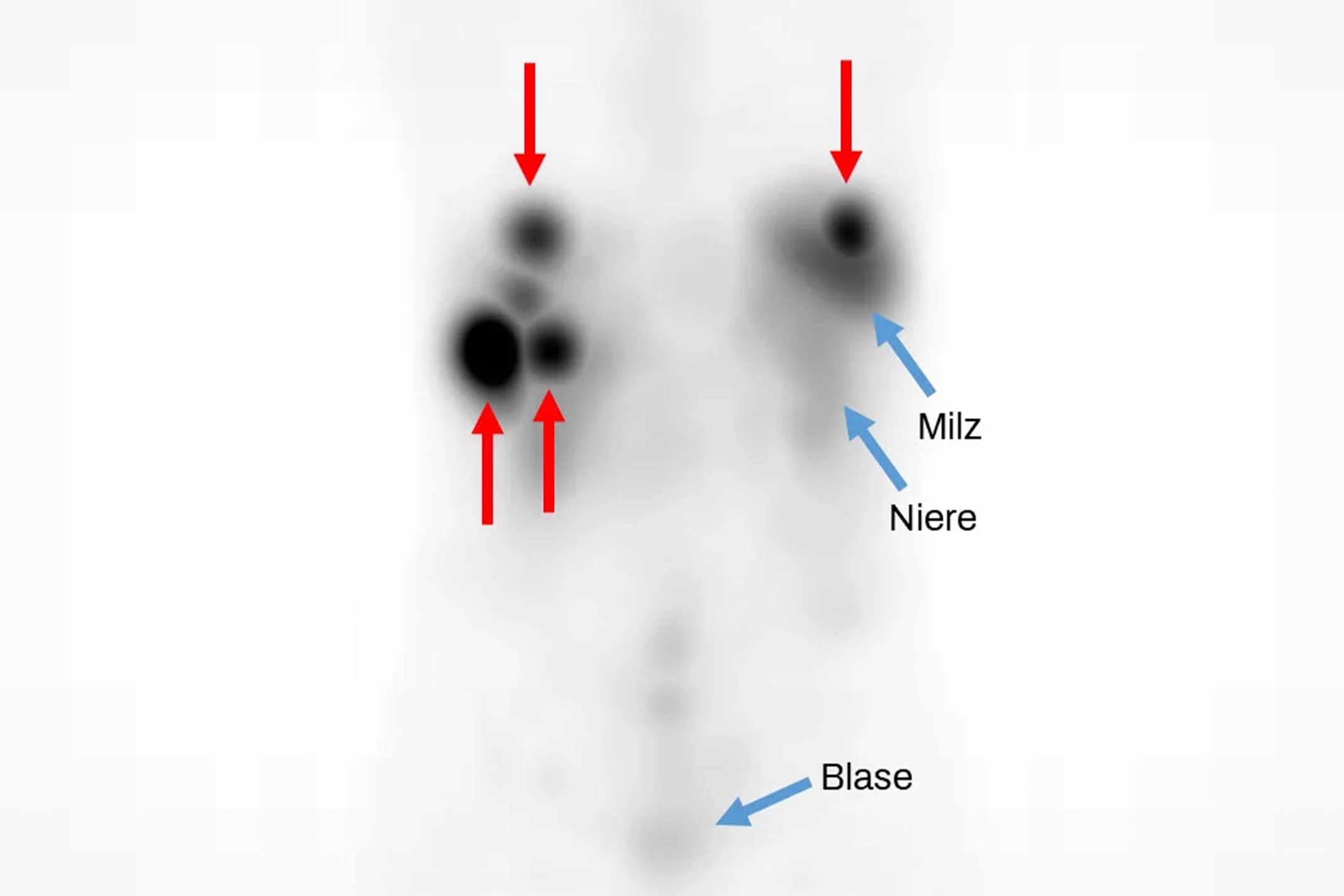
A promising radiopharmaceutical against metastatic neuroendocrine tumours – developed at the Paul Scherrer Institute PSI – has been successfully tested for the first time in patients and was featured as the “Image of the Month” in Europe’s leading journal for nuclear medicine. The encouraging results were published there by a research group at the University Hospital Basel.
Neuroendocrine tumours are a comparatively rare form of cancer originating in the cells that produce hormones. The University Hospital Basel has played a leading role in the research and treatment of this disease for several decades.
The clinical study that has now been published in the renowned European Journal of Nuclear Medicine and Molecular Imaging pursues a new approach to tackling metastatic neuroendocrine tumours. In future, patients who are not or no longer responding adequately to existing therapies could be given a substance labelled with the radioactive nuclide terbium-161. The new radionuclide terbium-161 was developed at the Paul Scherrer Institute PSI in Villigen, and the new radiopharmaceutical, 161Tb-DOTA-LM3, has undergone preclinical studies. The 161Tb-DOTA-LM3 for the current study is being made at PSI. 161Tb-DOTA-LM3 is now being tested in a clinical trial led by Damian Wild and Julia Fricke, both from the University Hospital Basel. The radiopharmaceutical accumulates selectively in the tumour cells and is said to destroy them more efficiently than the radiopharmaceutical currently used. In the first test subjects, the irradiation of the tumour cells was shown to be 9 times higher than using the previous standard treatment, which was developed 30 years ago – also at the University Hospital Basel.
The promising results of the study, which was funded by the Swiss National Science Foundation with a grant worth CHF 800,000, were featured by the EuropeanJournal of Nuclear Medicine and Molecular Imaging as its “Image of the Month”. The journal is one of the world’s two leading scientific journals for nuclear medicine research.
Text based on a press release from the University Hospital Basel
© PSI provides image and/or video material free of charge for media coverage of the content of the above text. Use of this material for other purposes is not permitted. This also includes the transfer of the image and video material into databases as well as sale by third parties.
Contact
Prof. Dr Roger Schibli
Head of the Centre for Radiopharmaceutical Sciences
Paul Scherrer Institute, Forschungsstrasse 111, 5232 Villigen PSI, Switzerland
+41 56 310 28 37
roger.schibli@psi.ch [German, English]
Prof. Dr Cristina Müller
Group Leader Nuclide Chemistry
Paul Scherrer Institute, Forschungsstrasse 111, 5232 Villigen PSI, Switzerland
+41 56 310 44 54
cristina.mueller@psi.ch [German, English]
Original publication
First-in-human administration of terbium-161-labelled somatostatin receptor subtype 2 antagonist ([161Tb]Tb-DOTA-LM3) in a patient with a metastatic neuroendocrine tumour of the ileum
Julia Fricke et al.
European Journal of Nuclear Medicine and Molecular Imaging, 07.03.2024
DOI: 10.1007/s00259-024-06641-w