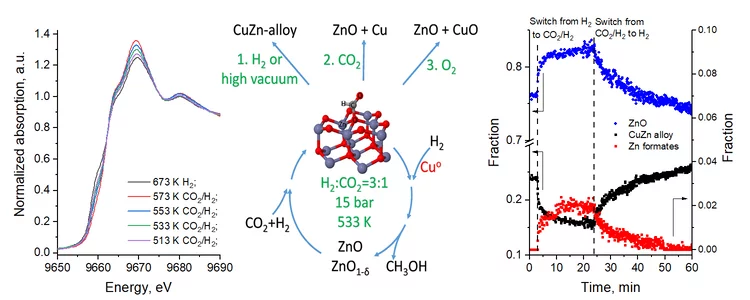
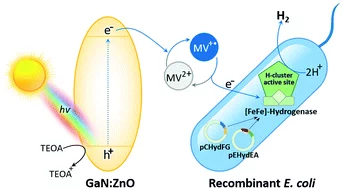
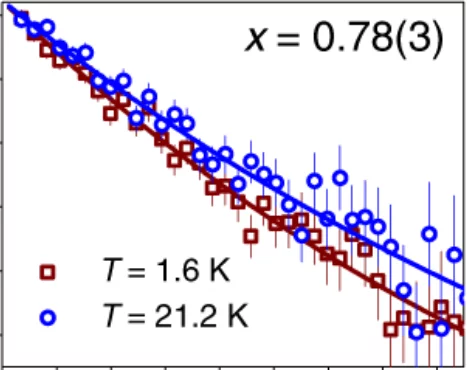
The energy research performed at PSI focuses on processes that can be used in sustainable and safer technologies, ideally with minimal CO2 emissions. The main emphasis is on renewable energy sources. The ESI (Energy System Integration) platform enables research and industry to test solutions for integrating renewables into the existing energy supply. Another focus in this area is the safer use of nuclear energy. These activities are supplemented by analyses giving a comprehensive assessment of energy systems. PSI scientists in the Energy and Environment division study the chemical processes that take place in the atmosphere.
Find out more at: Overview Energy and Climate