A Finnish-Swiss team cracks the atomic structure of a major cancer drug target
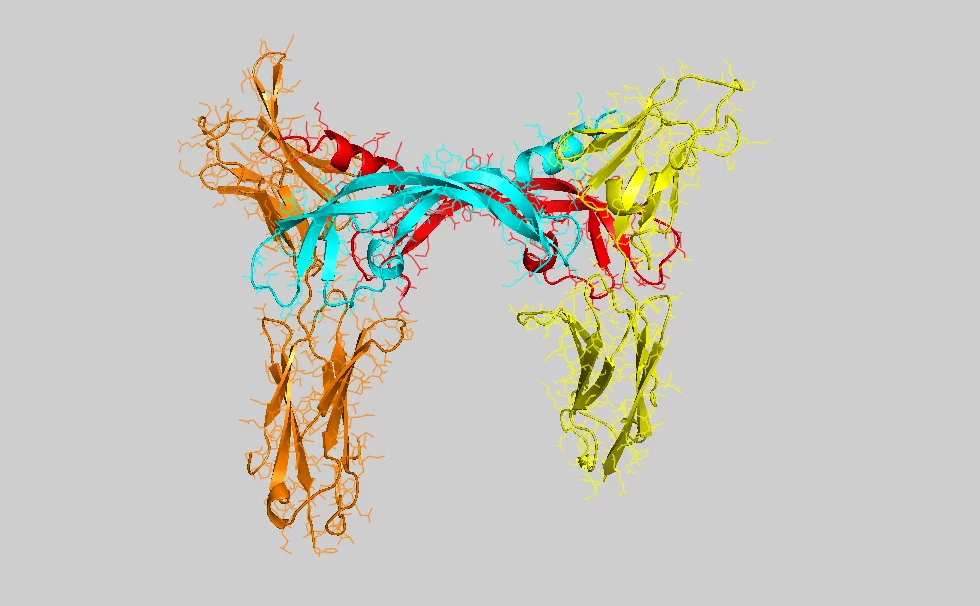
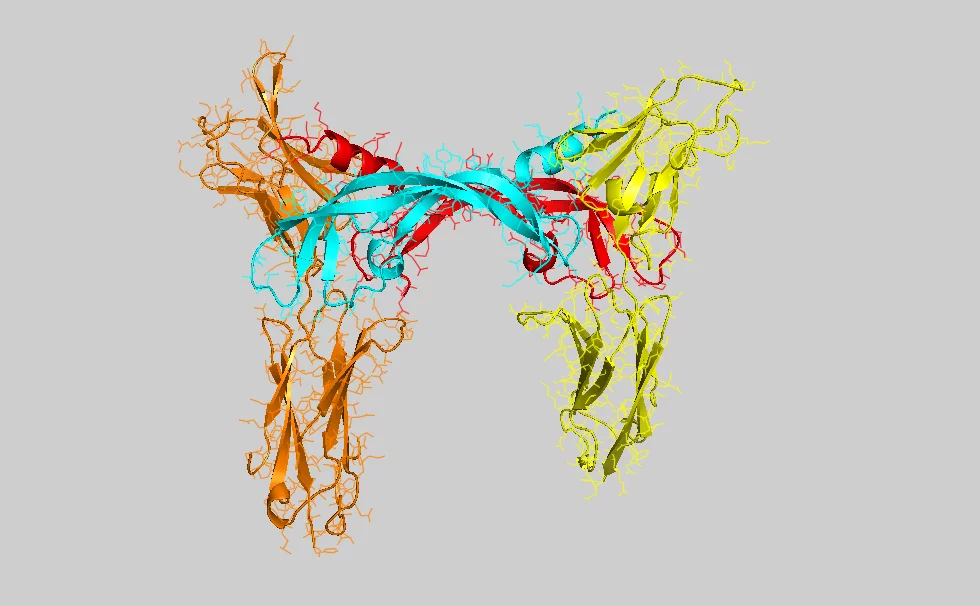
Researchers at Biomedicum Helsinki, Finland, and the Paul Scherrer Institute (PSI) in Villigen, Switzerland, have determined the crystal structure of the ligand-binding domain of a vascular endothelial growth factor (VEGF) receptor in complex with one of its ligands (VEGF-C).
Cancer cells require access to blood and lymph vessels for invasive growth and metastasis. By releasing VEGFs, cancer cells stimulate the surrounding blood vessels to invade the cancerous tumor mass. Blocking this process is a new strategy for inhibiting tumor growth. VEGFs and their receptors have been identified as major targets for drug development in cancer therapy, and the VEGF receptor that the groups analyzed is currently the most important target of such drugs. The Finnish group discovered the VEGF-C growth factor in 1996 and found that it is involved in lymphatic vessel growth, cancer metastasis and, more recently, also in blood vessel growth in cancer.
The collaborative work published in PNAS by the two research teams provides significant new insights into VEGF receptor function. It was also made possible by the long-standing interest and collaborative research of the binational teams and the availability of excellent crystallography beamlines at the Swiss Synchrotron Light Source, located at PSI.
About 20 years ago, American medical scientist Judah Folkman proposed a new tumour therapy through which a tumour was starved by the targeted blockage of the growth of its blood vessels and thus indirectly prevented from expanding. Treatment based on this concept has now been developed and is in clinical use. In order to optimise this method, it is important to understand the details of the molecular processes involved in the creation of blood vessels. The findings of Swiss and Finnish scientists reported here represent a decisive step forward in this area.
Experts use the abbreviation VEGF (vascular endothelial growth factor) to describe the molecules responsible for the growth of vessels. The molecule examined by PSI and Biomedicum, which particularly triggers the growth of lymph vessels, is called VEGF-C. From a biochemical perspective, a VEGF molecule is a protein, i.e. a highly complex biological molecule consisting of thousands of atoms. It becomes active when binding to another protein (the receptor) exposed on the surface, the plasma membrane, of cells. The binding of VEGF to the receptor causes a change in the structure of this membrane protein. Within seconds, the resulting structural change is transmitted across the membrane to the intracellular part of the receptor and triggers chemical reactions leading to the activation of the cells. In the case examined by the scientists, the cell is triggered to migrate and divide, and thus contribute to the growth of new blood and lymph vessels. Correct association of the signalling molecule with its receptor requires a unique molecular structure – i.e. thousands of atoms have to be correctly positioned in space. In order to determine this structure in detail, molecules were examined at the Swiss Synchrotron Light Source (SLS) at the Paul Scherrer Institute. This particle accelerator generates X-rays of unparalleled intensity, allowing, for example, the structure of complex proteins to be determined. The protein crystallography technique used in the study relies on the arrangement of individual molecules in a regular lattice (a protein crystal), which is then exposed to the X-ray source. Some of the beam is deflected in specific directions, allowing scientists to determine the detailed 3-dimensional structure of the protein.
The ongoing project at PSI which led to the PNAS publication is supported by PSI, the Swiss National Science Foundation (SNSF) and the Swiss Cancer League. The Finnish group was supported by the Sigrid Juselius Foundation, the Louis Jeantet Foundation, the Finnish Cancer Research Organizations, and the European Union integrated projects on Lymphangiogenomics, Tumor-Host Genomics, and MicroEnviMet.
About PSI
The Paul Scherrer Institute develops, builds and operates large, complex research facilities, and makes them available to the national and international research community. The Institute's own key research priorities are in the investigation of condensed matter and materials sciences, elementary particle physics, biology and medicine, and energy and the environment. PSI is Switzerland's largest research institution, with 1300 members of staff and an annual budget of approximately 260 million CHF.
Additional information:
Prof. Dr. Kurt Ballmer-Hofer, Laboratory for Biomolecular Research, Paul Scherrer Institute, 5232 Villigen PSI, SwitzerlandE-mail: kurt.ballmer@psi.ch; Telephone: +41 56 310 4165 [German, English]
Dr. Andrea Prota, Laboratory for Biomolecular Research, Paul Scherrer Institute, 5232 Villigen PSI, Switzerland
E-mail: andrea.prota@psi.ch; Telephone: +41 56 310 5160 [German, English, Italian, French]
Original publication:
Structural determinants of growth factor binding and specificity by VEGF receptor 2Veli-Matti Leppänen, Andrea E. Prota, Michael Jeltsch, Andrey Anisimov, Nisse Kalkkinen, Tomas Strandin, Hilkka Lankinen, Adrian Goldman, Kurt Ballmer-Hofer, and Kari Alitalo
PNAS Early Edition, January 18, 2010