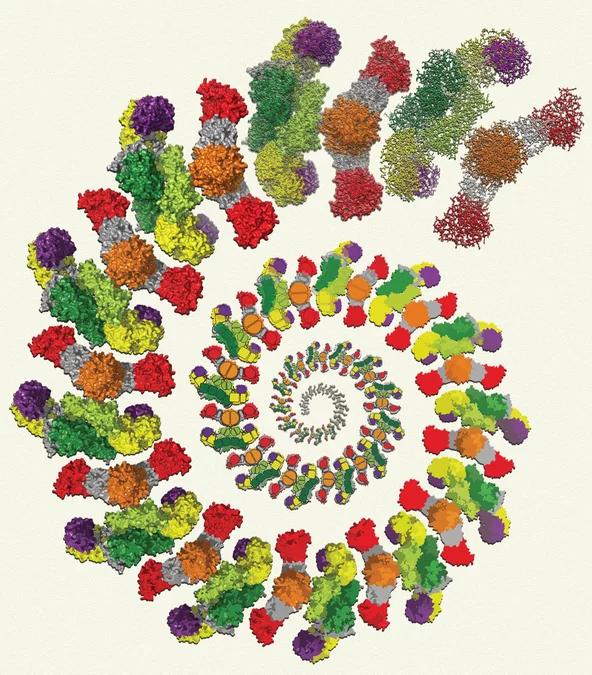
ETH Researchers determine atomic structure of the mammalian fatty acid factory
Zurich, 4 September 2008.
Mammalian fatty acid synthase is one of the most complex molecular synthetic machines in human cells. It is a promising target for the development of anti-cancer and anti-obesity drugs and for the treatment of metabolic disorders. Researchers of ETH Zurich have determined the atomic structure of a mammalian fatty acid synthase.
Synthesis of fatty acids is a central cellular process that has been studied for many decades. Fatty acids are used in the cell as energy storage compounds, messenger molecules and as building blocks for the cellular envelope. Previously, individual steps of this process have been investigated using isolated bacterial enzymes. However, in higher organisms – except plants – fatty acid synthesis is catalyzed by large multifunctional proteins where many individual enzymes are brought together to form a molecular assembly line
.
The atomic structure of a mammalian fatty acid factory
is the result of many years of research at ETH Zurich
As described in this week's issue of Science
magazine, researchers at ETH Zurich, supported by the National Centre of Excellence in Research (NCCR) in Structural Biology of the Swiss National Science Foundation, determined the high-resolution structure of a mammalian fatty acid synthase using data collected at the Swiss Light Source (SLS) of the Paul Scherrer Institute (PSI). These results crown the efforts towards determining detailed structures of fatty acid synthases in higher organisms, a milestone for future investigations, that a relatively small group of scientist at ETH Zurich, consisting of Timm Maier, Marc Leibundgut and Simon Jenni in the laboratory of Prof. Ban, have been pursuing for a number of years. This topic of research was initiated at ETH in 2001 and the first papers describing architectures of fungal and mammalian fatty acid synthases appeared two years ago in Science. Last year, the atomic structures of two fungal fatty acid synthases and the mechanism of substrate shuttling and delivery in these multi-enzymes were published in the same magazine. The latest paper in this series describes the atomic structure of the mammalian fatty acid synthase. These results reveal the details of all catalytic active sites responsible for iterative fatty acid synthesis and show how the flexibility of this large multi-enzyme is used for transferring substrates from one enzymatic active site to the next.
Fatty acid synthases as drug targets?
Besides the fundamental scientific interest in the function of this multi-enzyme with a central role in primary metabolism, mammalian fatty acid synthase is also considered a promising drug target. Although most fat accumulated in animals and humans is delivered to cells by ingestion and not by de novo synthesis, compounds that inhibit the function of the mammalian fatty acid synthase induce weight reduction in animals, showing potential for the treatment of obesity and obesity-related diseases, such as diabetes and coronary disorders. Furthermore, due to the increased requirement for fatty acid synthesis in cancer cells, inhibitors of this enzyme have anti-tumor activity, making fatty acid synthase an attractive drug target for anti-cancer therapy.
Multi-enzymes: the ultimate organic chemists
Mammalian fatty acid synthase belongs to a large family of multi-enzymes, some of which are responsible for synthesis of complex natural products of outstanding medical relevance with antibiotic, anticancer, antifungal and immunosuppressive properties. The structure of mammalian fatty acid synthase reveals how different catalytic domains are excised or inserted in various members of this family to yield multi-enzymes capable of synthesizing a large variety of chemical products. It facilitates the design of novel molecular assembly lines for the production of novel compounds. In particular, engineering of novel multi-enzymes for the production of modified antibiotics is important in the fight against resistant strains of bacteria.
Contact:
Clemens Schulze-BriesePaul Scherrer Institut, 5232 Villigen PSI, Switzerland
E-Mail: clemens.schulze@psi.ch; Phone: +41 56 310 45 33
Furter Information:
ETH Life: http://www.ethlife.ethz.ch/archive_articles/080905_Ban_Paper_Synthase/indexScience: http://www.sciencemag.org/cgi/content/abstract/321/5894/1315