Detailed structural information provides the foundation for new drugs
Anti-cancer drugs used under the heading of “Chemotherapy” prevent cells from dividing. As the cells in a growing tumour divide more frequently than others, tumour cells in particular are highly damaged by chemotherapeutic drugs. Scientists at the Paul Scherrer Institute and ETH Zurich have now elucidated the mechanism of action for an entire class of these drugs. These drugs “freeze” the motility of microtubules – fine fibres that are found in all cells – such that the newly-formed chromosomes are prevented from being distributed to the daughter cells. In this way, these drugs hinder cell proliferation. The scientists have shown in detail how these drugs are incorporated into a recess in the building blocks of microtubules, and reinforce the cohesion between these units. It has been shown that structurally dissimilar drug molecules could bind to the same site and act in a similar manner. The information obtained about these structures is so accurate, that it opens the possibility to develop targeted drugs that are better adapted to fulfil their task. One of the two drugs examined – a natural product occurring in a marine sponge, has been reproduced and synthesised at ETH Zurich. Its exact structure was determined at the Swiss Light Source SLS at the Paul Scherrer Institute. The results were published recently as an online pre-print in the journal Science.
In order for cancers to grow rapidly, their cells must divide more frequently. Drugs used as chemotherapeutic agents inhibit cell division by interfering with the cellular processes involved. One such process is the separation of chromosomes, the carriers of genetic information. During cell division the chromosomes destined for the two new cells, must be separated and transported to the new daughter cells. Microtubules are responsible for this task – these are fine fibres, that undertake a variety of functions in the cell, and to which the chromosomes are attached at the beginning of the cell division process. The microtubule mobility this demands arises from the fact that their individual components – molecules of the protein tubulin – are only loosely connected to each other. Thus new tubulin subunits can attach and detach easily at the ends of microtubules, such that the ends of the fibre can move through the cell. Certain cancer drugs impede the disassembly of microtubules, thereby restricting their mobility to the extent that they are no longer able to separate the chromosomes from each other. Thus, these drugs ultimately prevent cell division. One such drug that has been used clinically since the early nineties is called Taxol - found in the bark of the Pacific Yew tree from which it was first isolated in the early seventies. But the question that remained unanswered until now was the key molecular ‘How?’: how do Taxol and substances with a similar action prevent the breakdown of microtubules and restrict or completely suppress their mobility?
Drugs enhance bonding
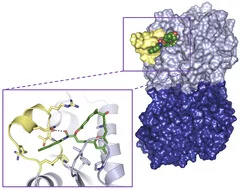
Scientists at the Paul Scherrer Institute and ETH Zurich have now elucidated the mechanism of action of two drugs that – like Taxol – stabilise microtubules: Zampanolide, a natural product originating in a deep-sea marine sponge, and Epothilone A, which is extracted from bacteria. Both substances are deposited in a cleft or “binding pocket“on the Tubulin surface. These stabilise a particular spatial arrangement of the region of the protein that links adjacent tubulin subunits. In this way, the cohesion between individual tubulin subunits is significantly enhanced. It is worth noting that both agents are from completely different biological sources and have chemically different structures – yet both fit into the “molecular binding pocket“ of tubulin and exert a similar effect.
Precise structure points the way to improved drugs
These results are important for developing better drugs. Because both the molecules studied work, but do not fit the binding pocket in tubulin perfectly. “For the first time, we have such accurate structural information about the binding site that we can develop targeted drugs that are optimally adapted to conditions in this binding site – and fit their structures like a hand in a matching glove“, says Andrea Prota from the Biomolecular Research Laboratory at the Paul Scherrer Institute.
Remaining challenges: drug molecule production, tubulin crystallisation
One of the anticancer agents has been synthesised by the Group of Karl-Heinz Altmann at ETH Zurich: “The synthesis of the Zampanolide molecule posed a huge challenge, and took nearly an entire doctoral dissertation to complete. Natural substances from marine organisms are as a rule only available in very minute quantities, and a detailed study of such substances can often be assisted by their synthesis in the laboratory. This was true in the case of Zampanolide,” says Altmann. The precise structure of the agent, together with the surrounding “binding pocket” in the tubulin molecule was then determined by researchers using the Swiss Light Source SLS at the Paul Scherrer Institute by employing a technique called protein crystallography. With this, the protein to be studied is first produced in large quantities and then arranged into an ordered crystal, (with the ‘binding pocket’ either empty or filled with a compound). This protein crystal is then illuminated with synchrotron light from the SLS. From the way in which the light is diffracted as it moves through the crystal, the structure of the protein can be deduced down to the last detail. “Tubulin molecules have a strong tendency to combine together to form fibres – they are in a crystalline state, but not exactly” said Michel Steinmetz, Head of the Protein Interactions Working Group at PSI. “So for this experiment, it was a big challenge to manipulate the tubulin such that its molecules lined up in a highly ordered crystal – a venture that has taken us more than 10 years of effort!“.
Text: Paul Piwnicki
About PSI
The Paul Scherrer Institute develops, builds and operates large, complex research facilities, and makes them available to the national and international research community. The Institute's own key research priorities are in the investigation of matter and material, energy and the environment; and human health. PSI is Switzerland's largest research institution, with 1500 members of staff and an annual budget of approximately 300 million CHF.
About ETH Zurich
ETH Zurich has come to symbolise excellent education, groundbreaking basic research and applied results that are beneficial for society as a whole. Founded in 1855, ETH Zurich has more than 17,000 students from approximately 80 countries, 3,700 of whom are doctoral candidates. Today, it offers researchers an inspiring environment and students a comprehensive education as one of the world’s leading universities for technology and the natural sciences. 21 Nobel Laureates have studied, taught or conducted research at ETH Zurich, underscoring the excellent reputation of the institute.
Contact
Prof. Dr. Michel Steinmetz, Paul Scherrer Institute,Research Group Dynamic protein interactions, 5232 Villigen PSI, Switzerland
Phone: +41 56 310 4754, E-mail: michel.steinmetz@psi.ch [German, English, French]
Dr. Andrea Prota, Paul Scherrer Institute,
Research Group Dynamic protein interactions, 5232 Villigen PSI, Switzerland
Phone: +41 56 310 5160, E-mail: andrea.prota@psi.ch [German, Italian, English]
Prof. Dr. Karl-Heinz Altmann, ETH Zurich
Institute of Pharmaceutical Sciences; 8093 Zürich, Switzerland
Phone: +41 44 6337390; E-mail: karl-heinz.altmann@pharma.ethz.ch [German, English]
Original Publication
Molecular Mechanism of Action of Microtubule-Stabilizing Anticancer AgentsAndrea E. Prota, Katja Bargsten, Didier Zurwerra, Jessica J. Field, José Fernando Díaz, Karl-Heinz Altmann, and Michel O. Steinmetz
Science, 3 January 2013 DOI: 10.1126/science.1230582