For the discovery and characterisation of the miraculous material graphene – a layer of carbon exactly 1 atom thick– two Russian born physicists were awarded the Nobel Prize in 2010 and got a huge amount of media attention. Graphene is an extraordinary material in many ways: it has a tensile strength about a hundred times higher than steel, yet a hypothetical area of 1 square kilometre weighs only one kilogram; its extremely high electrical conductivity makes it a candidate for the next generation of transistors. The list of its advantages would go on for several pages. Because of this, ever since graphene was first isolated, scientists all over the world have been rushing to find applications. Recently, scientists at the Paul Scherrer Institute PSI laid the foundations for a graphene-based super capacitor. With its help, the lifespan of batteries in hybrid cars could be extended significantly.
Supercaps – energy storage for a quick kick
Supercapacitors – also known as supercaps or ultracaps in the professional world – are energy storage devices like batteries, but their operation is based entirely on electrostatic forces. This means: unlike batteries, no chemical reactions are involved in the game here. Instead, electrolyte ions are bound to the electrode surface by attractive electrostatic forces, thereby forming a double layer that is just a few nanometres thick. From these, the supercaps’ technical name “double layer electrochemical capacitors” is derived. By switching the voltage applied to the electrodes on and off, the supercaps can be charged and discharged very rapidly. This is the basis of their high power density, that is, their ability to release or accept large quantities of energy in a very short time period.
Supercaps have been produced for applications like “regenerative braking“, which allows recovery and storage of energy that would otherwise be lost in braking in hybrid cars. This is because the process involves receiving a lot of energy in a fraction of a second - a task batteries can only perform with appreciable wear and a corresponding decrease in their lifespan. This is the reason many car manufacturers find supercaps desirable - they can store energy rapidly and spare the batteries. Others already have supercaps on board, which likewise supply the rapid automatic start/stop systems of diesel vehicles with energy. Another area of application for supercaps is for regulating safe shutdown in wind turbines, which have to be shutdown in a matter of minutes during stormy weather to avoid structural damage. Also the emergency doors on passenger aircraft have to open quickly and reliably: those on the Airbus 380 already handle this by using supercaps.
Super caps also stand out in terms of longevity: because they do not rely on chemical reactions, there is virtually no wear on the electrode materials. A supercapacitor can be charged several million times without undergoing any significant impairment. For most purposes, this results in a lifespan of 10 to 20 years- in contrast to the 2 to 3 years of the best accumulators.
Activated carbon has become the established supercap electrode material over the past decades. Due to its branched structure, the pores in just two or three grams of activated carbon cover an area the size of a football pitch. The enormous available surface area provides ample space for the docking of many ions, however, the labyrinthine structure of activated carbon means this surface area is only partially accessible. Thus the potential of activated carbon as a storage material is limited, and the search for alternatives is going on in many laboratories across the world.
A graphene electrode for high performance super caps
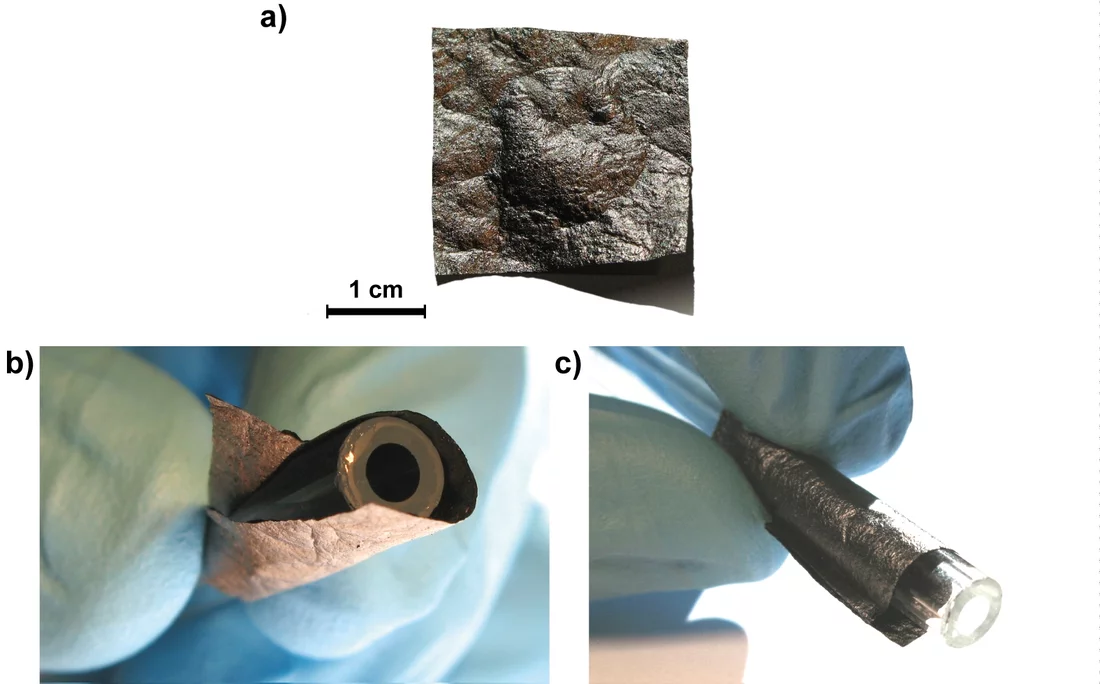
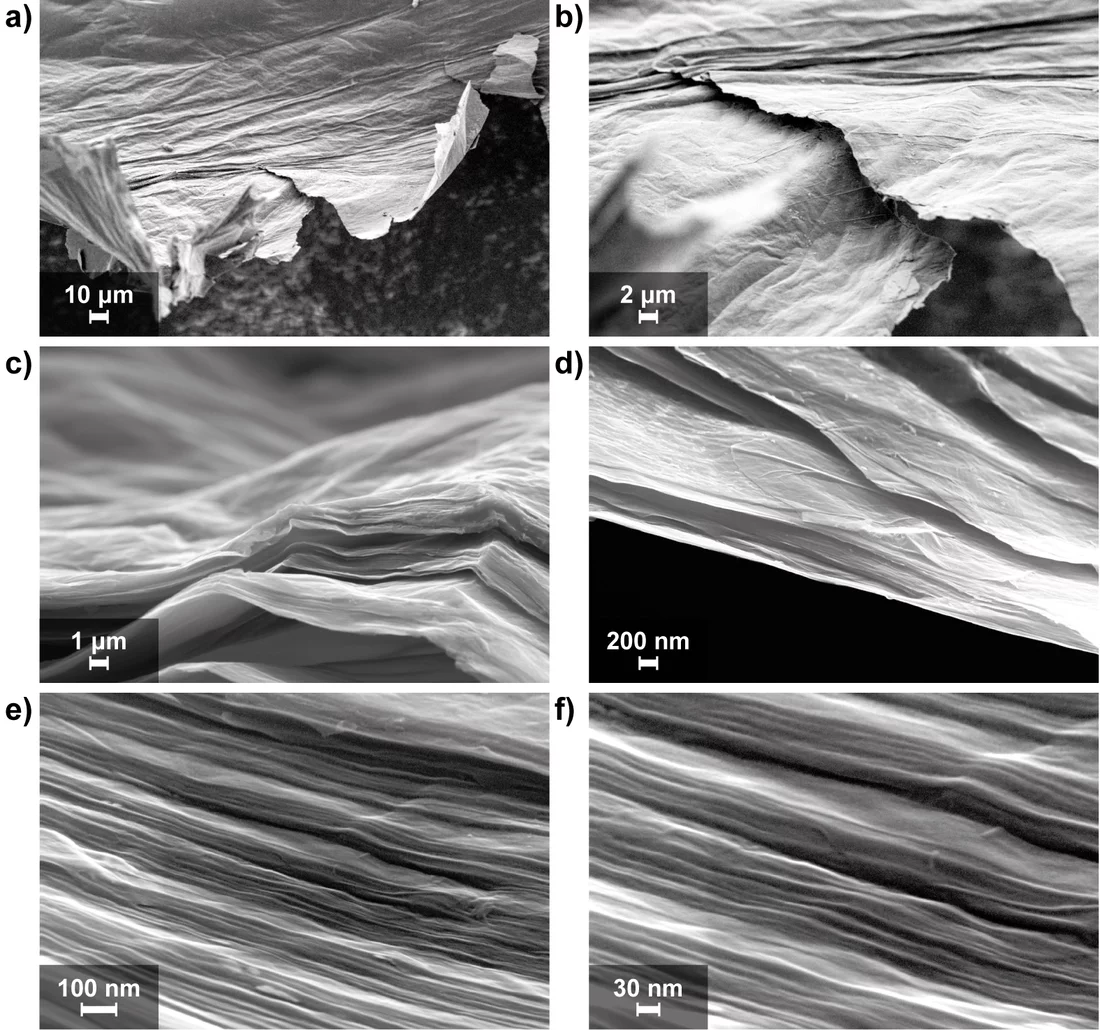
Scientists at the PSI have pinned their hopes on graphene. Their choice has been based on the fact that graphene offers the highest theoretically achievable surface area per unit weight for a carbon-based material, which, for increasing the storage capacity of super capacitors, is the deciding factor. Ph.D. student Moritz Hantel and his supervisor Rüdiger Kötz, together with the research group of Reinhard Nesper from ETH Zurich have produced and characterised a graphene based electrode which appears highly promising for use in supercaps, In their “graphene paper,” storage takes place on flat layers stacked on top of one another, instead of the sponge-like labyrinthine passages of activated charcoal. To produce these “graphene sheets” the scientists employed a special filtration process (“flow-directed filtration”). In this, graphite oxide is first dispersed in water, then broken up into individual layers (graphene oxide) with the help of ultrasound. This graphene oxide-dispersion is then filtered through a filter paper (“suction-filtered”). During filtration the individual graphene layers are randomly oriented on top of one another and thus produce a paper-like structure of overlapping graphene layers.
The graphene oxide paper synthesised this way provides a lot of space for the docking of electrolyte ions, but would be useless in a capacitor. The reason being its low electrical conductivity, which is ultimately the critical determinant of the discharge of mobile electrons (electric current). To impart a higher electrical conductivity to the graphene paper, the material is subjected to “partial thermal reduction”. This is achieved by controlled heating of the graphene oxide membrane, and results in both the carbon scaffolding being reduced as well as some sections of it being oxidised to CO and CO2. As a result, the carbon-structure gains significant conductivity. In comparison to normal “chemical reduction”, one advantage of “thermal reduction“ is that the impurities formed in the former are avoided. The other advantage is that previous studies have demonstrated thermally-reduced graphene oxide has a superior conductivity to its chemically-reduced counterpart.
At the end of the process, one has an outstanding material for ion storage. The enhanced ability to store and release ions arises because the distance between the graphene layers has almost doubled in comparison to the starting material. Exactly how this takes place however is unknown. A chemical reaction appears to take place between the organic electrolytes and graphene, which researchers have suggested produces a sort of solid ”pillar“, that keeps the graphene sheets a fixed distance apart. Due to this increased separation, ions can enter and leave the empty space between graphene sheets more easily. Since the area between the sheets cannot be measured, scientists cannot make a comparison between the specific surface area of their material and activated carbon. However, it is clear that the specific capacity of their graphene electrode, at about 200 Farad (the unit for measuring electrical capacity) per gram, is amongst the highest to be found in the scientific literature.
Scientists are currently working towards finding an explanation for the expansion of the graphene structure, which allows its increased storage capacity. “Demonstrating the existence of the posited “pillar” structures between graphene sheets may be possible within the next few months,” said Hantel. This should be followed by additional investigations aimed at understanding the factors that may influence the longevity of the material. This should open up an exciting new chapter in the success story of the ultrathin carbon material graphene.
Text: Leonid Leiva
Original publication
Partially Reduced Graphene Oxide Paper: A Thin Film Electrode for Electrochemical Capacitors, Moritz M. Hantel, Tommy Kaspar, Reinhard Nesper, Alexander Wokaun and Rüdiger Kötz, J. Electrochem. Soc. 2013, Volume 160, Issue 4, Pages A747-A750. (DOI: http://jes.ecsdl.org/content/160/4/A747)Contact
Dr. Rüdiger Kötz, Head of the Group for electrocatalysis and interfaces, Paul Scherrer Institute,Telephone: +41 56 310 20 57, email: ruediger.koetz@psi.ch