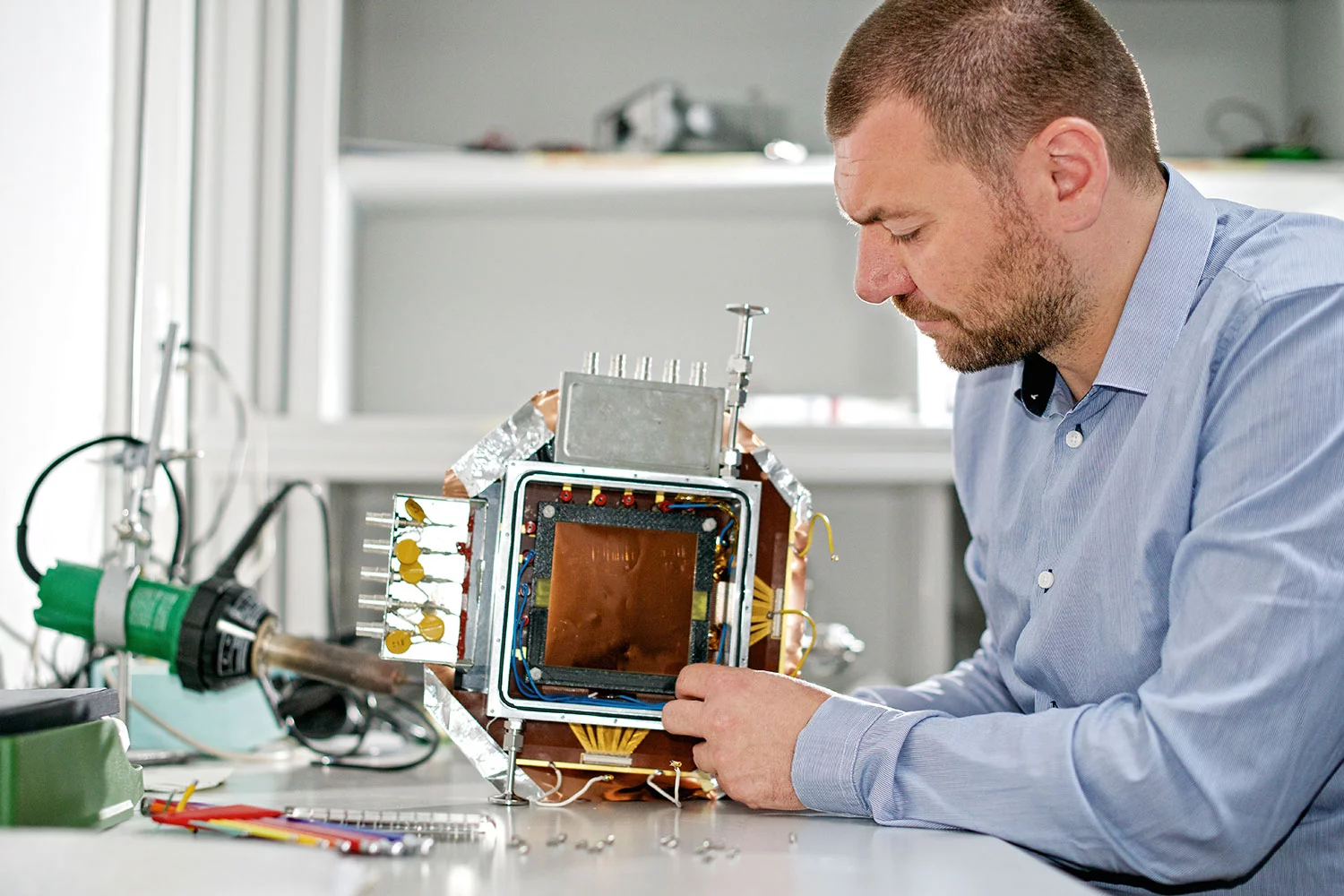
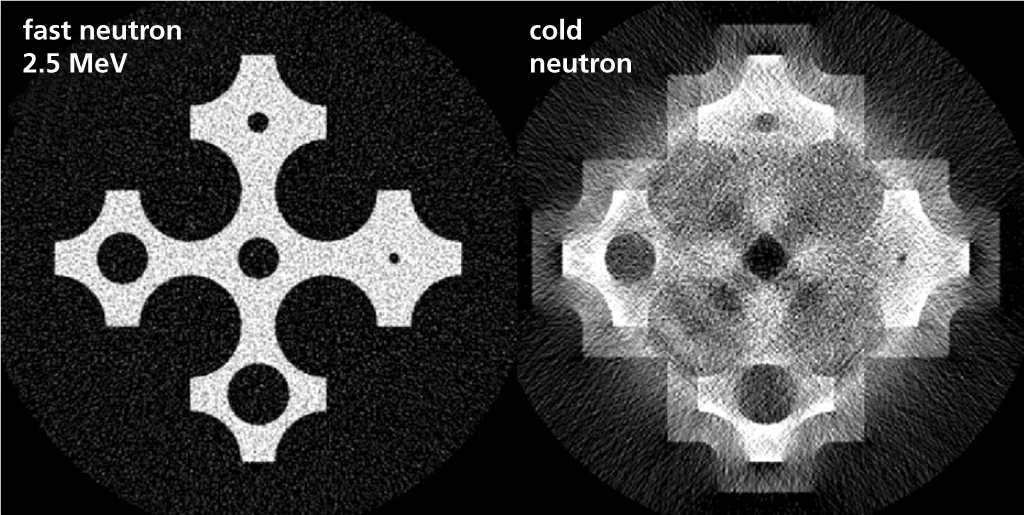
Neutrons are an excellent tool for the non-destructive imaging of the interior of objects. For some materials that are virtually opaque or cannot be distinguished by X-rays, neutrons provide the only informative ‘dissection tool‘. However, neutron radiography is confined mainly to the laboratory and to fixed facilities, because neutron generation relies on equipments which are costly, complex and cannot be moved. Scientists at the Paul Scherrer Institute PSI want to develop a more flexible, alternative imaging technique based on fast neutrons.
Neutrons are together with protons the building blocks of all atomic nuclei (except for the lightest form of the hydrogen atom). Unlike the protons, they are electrically neutral (no charge) and they are also slightly heavier. As free particles outside the atomic nucleus, neutrons are unstable, decaying with a mean lifetime of almost 15 minutes. Free neutrons are obtained from nuclear reactions, usually out of nuclear reactors or from spallation sources, in which protons from a particle accelerator are shot onto a heavy metal target (e.g. lead) producing neutrons as a result of the fragmentation of the atomic nuclei.
In contrast to X-rays, neutrons are absorbed most strongly by organic materials and elements with low atomic number. Neutrons are therefore particularly suitable for imaging materials with a high hydrogen content. For such samples, unlike radiographic images, neutrons can provide information about their density, as well as about the exact distribution of their chemical components.
However, neutron radiography in the laboratory is confined to fixed installations, since neutron generation relies on equipment like nuclear reactors or particle accelerators, which are costly, complex and cannot be moved. To date, neutron imaging has been established using so-called cold or thermal neutrons which have only moderate energies. Scientists at the Thermal-Hydraulics Laboratory at the Paul Scherrer Institute (PSI) are developping an imaging technique based on fast neutron. The neutron imaging system currently under construction is comprised of a compact neutron source and neutron detectors, suitable for imaging, are developed in parallel. In particular, the main components of the detector can be produced from low cost materials like polyethylene plastic. Hence neutron imaging in safety related environments could become portable, and useful in the field.
Back to the source
The first challenge is the production of fast neutrons. The PSI-scientists addressed this by using deuterium atoms. Deuterium is a heavier isotope (form) of hydrogen, and has like hydrogen an electron and one proton but it has also an additional neutron in its nucleus. It is this neutron that the scientists want to knock out of the nucleus at high speed. To do this, they use a deuterium containing plate as a target, which is bombarded with deuterium atoms. When one deuterium atom, acting as a projectile, meets a deuterium atom on the target, a nuclear fusion reaction takes place, in which neutrons with a high speed (and therefore high energy) are emitted. Thanks to this method, it is possible to build a neutron source that is only 2 mm wide, which produces a neutron beam that provides with a sufficient spatial resolution for a wide range of applications.
How do you detect neutrons?
With a neutron beam to illuminate through samples, one also needs a detector to measure the neutrons transmitted through that sample. To construct an efficient neutron detector is as difficult a task as building a good source. Neutrons are not electrically charged, and are not influenced by electromagnetic forces. That is why they can penetrate samples easily. But it’s also why they are so hard to detect. Neutron detection is based on the reaction of neutrons with the atomic nuclei in the sample material. In these reactions, neutrons will either be absorbed or deflected from their original trajectory.
In the detector developed by the PSI researchers, a plastic (polyethylene) film serves as the first element in the detection chain, where the neutrons transfer a part of their energy to protons by colliding with hydrogen atoms (protons) of the plastic. Polyethylene is an extremely hydrogen rich material and the neutrons act like a billiard cue knocking out the hydrogen “billiard balls” from the plastic. These protons then in turn, knock out electrons (ionise) in a neon-containing gas mixture. The electrons released are eventually multiplied and deflected onto the sensors by a strong electric field. The trajectories of the electrons in the electric field is known and well defined, which allows the scientists to reconstruct a two-dimensional image of the illuminated objects.
Measuring the energy, to “see” the chemical composition
Using fast neutrons, the scientists not only want to image these samples, but also to elucidate their chemical composition. To do this, the energies of the neutrons that reach the detector must be measured. This energy spectrum is like a fingerprint of the sample, since every chemical element slows or deflects neutrons with a different intensity, which means that neutrons leaving the sample reach the detector with different intensities at different energies, depending on which elements they come into contact with. From the measured neutron energy spectrum, one can deduce which chemical elements the sample is made of.
To measure neutron energies, the scientists have designed a detector that can be thought of as a ’step filter‘. The protons, knocked out by the neutrons, are filtered out at different positions along the detector according to their kinetic energy. Lower-energy protons are stopped (filtered) already near the surface of the detector facing the object. Whereas faster (higher energy) protons will be filtered only deeper within the detector. To stop the protons, for example, thin aluminium foils are pasted onto the back of the polyethylene layers. The “step filter” effect is reached by gradually increasing the thickness of this stopper layer along the depth of the detector. In this way only protons with a certain threshold (minimum) energy will survive at each stopper and the rest will be absorbed by the ever thicker stopper layers.
No hide and seek for explosive materials
With the ability to determine the chemical composition of a sample, scientists working with safety authorities now have a very useful tool.Dangerous items hidden in a suitcase or container can now be identified by more than just their physical shape. A firearm can be revealed from its outline, but a bomb can be hidden inside a seemingly harmless container. Since explosives can be clearly identified by their chemical signatures, with this new technology, they could not even remain disguised behind a simple soft-drink bottle. As fast neutrons can penetrate more easily large volumes than cold or thermal neutrons, they could also be used to provide better images of the inside of a suspicious ship container or truck.
Besides potential security applications scientists aim to contribute to improved, safety of nuclear power plants using fast neutron imaging techniques. In particular, they look at problems involving larger quantities of water, like the cooling processes in nuclear fuel bundles. Water is one of the best available neutron stoppers and larger amounts of water would absorb and stop entirely neutrons with low energy. However fast neutrons are not so easy to stop and can penetrate through larger amounts of water delivering still information about the process.
Investigating fast, dynamic processes is also aimed at by the researchers. That includes processes like the evaporation of the layer of cooling water around the fuel rods of an overheated nuclear fuel bundle. Using a pulsed source of fast neutrons, such processes could be captured in much higher details than so far possible. This could result in an optimised design of fuel rods for nuclear reactors.
Text: Leonid Leiva
Additional information
Laboratory for thermal hydraulicsResearch with cold and thermal neutrons at PSI