In nighttime photographs taken from space, the large cities of the world can easily be recognised by the flood of their public lighting. However, probably only the trained eye is able to see, as well as New York or Tokyo, the locations of many oil-producing wells . The light in these cases originates mainly from the combustion of methane. This huge waste of an energy-rich gas has devastating economic and ecological consequences. Reasearchers at the Paul Scherrer Institute PSI are looking for a solution: the conversion of methane into the liquid energy carrier methanol
Methane is often found in crude oil reservoirs, and escapes during oil production because of its low density – in the same way that carbon dioxide rises up and fizzes out of an opened soda bottle. Because, at current market prices, the processing, storage and transport of methane would be too expensive, the gas from oil extraction is either burned immediately – which produces CO2 - or simply released into the atmosphere. Through these so-called flaring or venting processes, 140 billion cubic meters of methane were thrown into the atmosphere surrounding our planet in 2011, according to World Bank statistics. This corresponds to 30 per cent of the annual methane consumption of Europe, and 75 per cent of the annual methane exports in Russia. Economically, the scale of the wasteful usage of this valuable natural resource is huge. The negative consequences are also environmental in nature. There is a massive deterioration in local air quality, and -when the gas is discharged directly into the atmosphere, there is a considerably sized contribution to climate change, since methane is a much more potent greenhouse gas than carbon dioxide.
Liquids win
Jeroen van Bokhoven, Director of the Catalysis and Sustainable Chemistry Laboratory at the Paul Scherrer Institute PSI envisions a solution to this enormous environmental and economic sin: the production of methanol from methane, right at the source, could transform the volatile gas into an easier-to-handle fuel. Storage and trasport to the consumer as methanol would be both technically and economically viable.
Methanol offers several advantages over methane. Firstly, at ambient temperature and normal pressure, it is in a liquid state. In addition, because of its molecular structure, methanol is more reactive than methane and thus forms a better starting material for the production of fuels and other useful chemicals. Thus far, however, chemists have found the efficient recovery of methanol from methane a hard nut to crack. Conventional production methods are too costly.
The conventional process for methane conversion includes, as its first step, the so-called steam reforming of methane gas. During steam reforming, methane is reacted with water vapour at a temperature of at least 700 degrees Celsius. This process step uses a huge amount of energy. From reforming, a mixture of carbon monoxide and hydrogen is produced, which is known as Syngas. This Syngas can then be further converted into methanol by chemical reactions at high pressure.
A more direct and less energy intensive conversion of methane to methanol is desirable, but has proved a stumbling block for fundamental reasons. With its symmetrical molecular structure, and because of the strong bonds between the carbon atom and the surrounding hydrogen atoms, methane is chemically very stable, which means it can be difficult to convert it to methanol without total oxidation to CO2. This problem can be circumvented by using catalysts - substances that can promote and accelerate chemical reactions without being consumed themselves. However, the search for the right catalyst for methanol production has remained fruitless, despite decades of effort.
Thermodynamics stand in the way
Methanol production as the end product of methane conversion goes against the “Will of Nature”. Or, to put it in more technical terms: the laws of thermodynamics that govern the conversion of materials in terms of energy minimisation favour complete oxidation of methane resulting in the production of carbon dioxide. To obtain methanol, one would have to use a trick to stop the oxidation process at the right time. To achieve this, chemists use what are called selective catalysts. These are substances that help get the reaction over the energy barrier for methane to methanol, but prevent it from going further to form CO2.
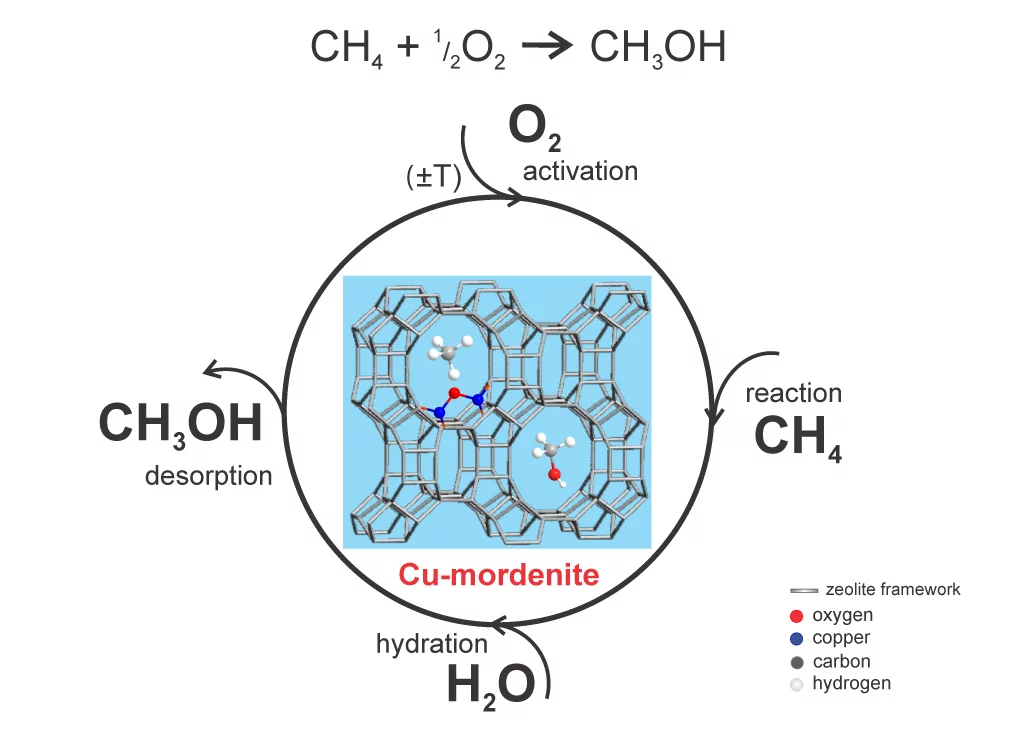
Scientists only achieved the catalytic conversion of methane to methanol a few years ago. As catalyst, they used a copper-impregnated zeolite, called copper-mordenite. Mordenite is a naturally occuring crystalline mineral made of aluminim, silicon and oxygen but is also chemically synthesisable.. Metal atoms, such as copper, incorporated into the pores of the crystal structure of the zeolite act as particularly efficient selective catalytic centres.
In these first attempts, production of methanol from methane with mordenite was complicated because the methanol produced was strongly absorbed on the surface of the copper-zeolite. After reaction with methane, the catalyst was extracted from the reactor and a solvent was introduced to separate out the methanol. The result was that the copper-zeolite was used only for one methane to methanol conversion. In addition, the scientists estimated that only a few percent of the copper atoms in the zeolite actually had a catalytic effect.
Scientists at the PSI have been building on this work, and have for the first time developed a process in which the methanol leaves the reactor as a final product without the use of solvents. They got the problem of methanol desorption under control. To reactivate the copper centres in the catalyst, they introduced oxygen to the reactor so that the copper centres are again active for a new methane conversion cycle. “In this way, we can achieve many conversion cycles before the catalyst has to be replaced“, said scientist Evalyn Mae Alayon, who developed the process as part of her Ph.D Dissertation. They also found out that there is a large fraction of copper centers that are reacting with methane.
During the development and optimisation of this new production method, PSI-scientists have benefited from the outstanding large-scale infrastructure that the PSI has at its command. Thus, using X-ray absorption spectroscopy studies at the SuperXAS beamline at the Swiss Light Source SLS, they were able to demonstrate the success of their process by relating structural changes of the copper centres to their catalytic activity and therefore to the evolution of the methane to methanol conversion reaction. In the future, they want to further improve the design of the catalyst using these X-ray techniques. Jeroen van Bokhoven is excited about the prospects of this. However, their success to date is already a source of pride, as achieving the catalytic conversion of methane to methanol has long been considered as a highly desireable “golden reaction” amongst chemists. If an industrialised reality results from it, the end of sheer waste of methane worldwide could happen, to some extent, thanks to the hard work of PSI-scientists.