A novel polymer electrolyte membrane from the Paul Scherrer Institute PSI has demonstrated longer durability in a laboratory test than the best commercially available counterparts. The breakthrough was achieved by modifying a reasonably priced plastic film through radiation activation and subsequent attachment of functional constituents via a “grafting” reaction. The modified polymer is not only durable – it could also reduce the membrane production costs by 50 to 80 percent. The membrane could be used in applications such as hydrogen fuel cells or electrolysers for hydrogen production from water.
Hydrogen fuel cells, like the ones being researched and developed at PSI, could turn the goal of motorised mobility with no impact on the environment into reality. In cars these cells would be used for propulsion and the only exhaust product would be water. However, there are still a few technical and economic hurdles in the way of that dream. One key issue is the durability of the cell that operates under harsh conditions, in particular the polymer membrane used as electrolyte and separator. Furthermore, the production costs would have to drop markedly if the fuel cell is to gain a foothold on the highly competitive automotive market. Researchers at PSI have moved closer to both of these goals. They have developed a polymer membrane that need not fear comparison with the best counterparts on the market.
Teflon and polyethylene in one
The starting material for the PSI membrane is the plastic ETFE (ethylene tetrafluoroethylene). Like every polymer it has a backbone consisting of a long chain of carbon atoms. Chemical compounds are attached to the backbone and they give the polymer its special characteristics. In the case of EFTE the compounds that alternate along the chain constitute the plastics polyethylene and Teflon. ETFE combines the advantages of these two plastics without any of the disadvantages. It is as easy to process as polyethylene and as chemically and thermally stable as Teflon.
For electrochemical applications ETFE must be modified. In particular ion conductivity nees to be introduced and its gas permeability minimized. Every material used, for instance, as the electrolyte membrane in a hydrogen fuel cell must have both these properties. In this kind of fuel cell the hydrogen ions (protons) migrate from the fuel electrode to the air electrode to react with oxygen to generate water and power. The hydrogen and oxygen may not come into direct contact with one another. Otherwise, this could lead to an uncontrolled thermal recombination reaction. That’s why the cell needs a membrane which allows protons to pass but prevents the passage of gases.
Competitive edge through radiation chemistry
ETFE is modified using a process that researchers call “radiation grafting”. In horticulture a valuable but weak plant is grafted to the base of a more robust one. In a similar fashion, the ETFE backbone serves as the base onto which the chemical compounds that impart the ETFE with ionic conductivity are grafted. A styrene derivative is attached ("grafted") as side chains onto ETFE. Sulphonation of the styrene units introduces an acid group; it gives ETFE the desired ion conductivity. Incorporating methacrylonitrile as a comonomer improves the gas barrier properties of the membrane. Prior to grafting the ETFE backbone must, however, be rendered receptive to the grafted component. For this purpose, the polymer is irradiated with electrons. The radiation leads to statistical bond scission events along the polymer chain, which leads to the formation of radicals at these breaking points. Because the radiation is performed in air, peroxides may likewise be formed that react readily and rapidly, too. These active centres and the radicals then serve as starting points for the actual “grafting” reaction.
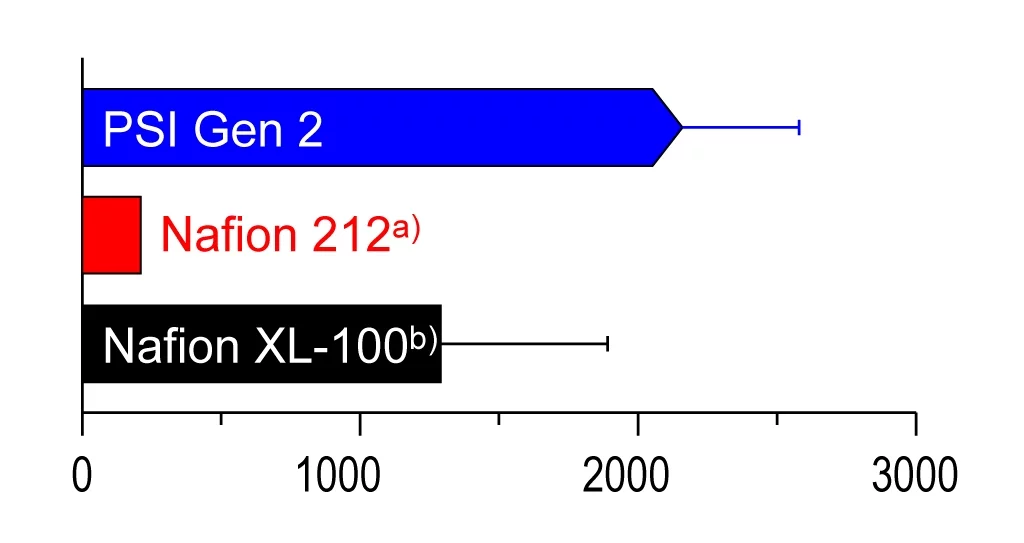
Longer lifetime than commercially available membranes
The refined ETFE membrane has proved its worth in durability tests in the laboratory. Two out of three membranes were still intact after 2,400 hours of test operation in typical stress cycles for vehicles. This means that the PSI membrane is superior in terms of longevity to the best membranes available on the market made of the polymer Nafion.
Besides its superior stability, the potential substantial low-cost fabrication method make the PSI membrane a very attractive candidate for a fuel cell membrane. Since the procurement costs for ETFE are far lower than for Nafion, the typical market option for membranes of this kind, a cost reduction of 50 to 80 percent is expected in the case of optimised industrial production. In the longer term, if the production volume increases correspondingly, the production costs would even fall by as much as 90 percent according to a study by the American company General Motors.
Text: Leonid Leiva
Contact
Dr. Lorenz Gubler, Head of the group Membranes and Electrochemical Cells,Electrochemistry Laboratory, Paul Scherrer Institute,
Telephone: +41 56 310 26 73, E-mail: lorenz.gubler@psi.ch