With the X-ray laser SwissFEL, researchers at PSI want to produce movies of biomolecules in action. This can reveal how our eyes function or how new drugs work.
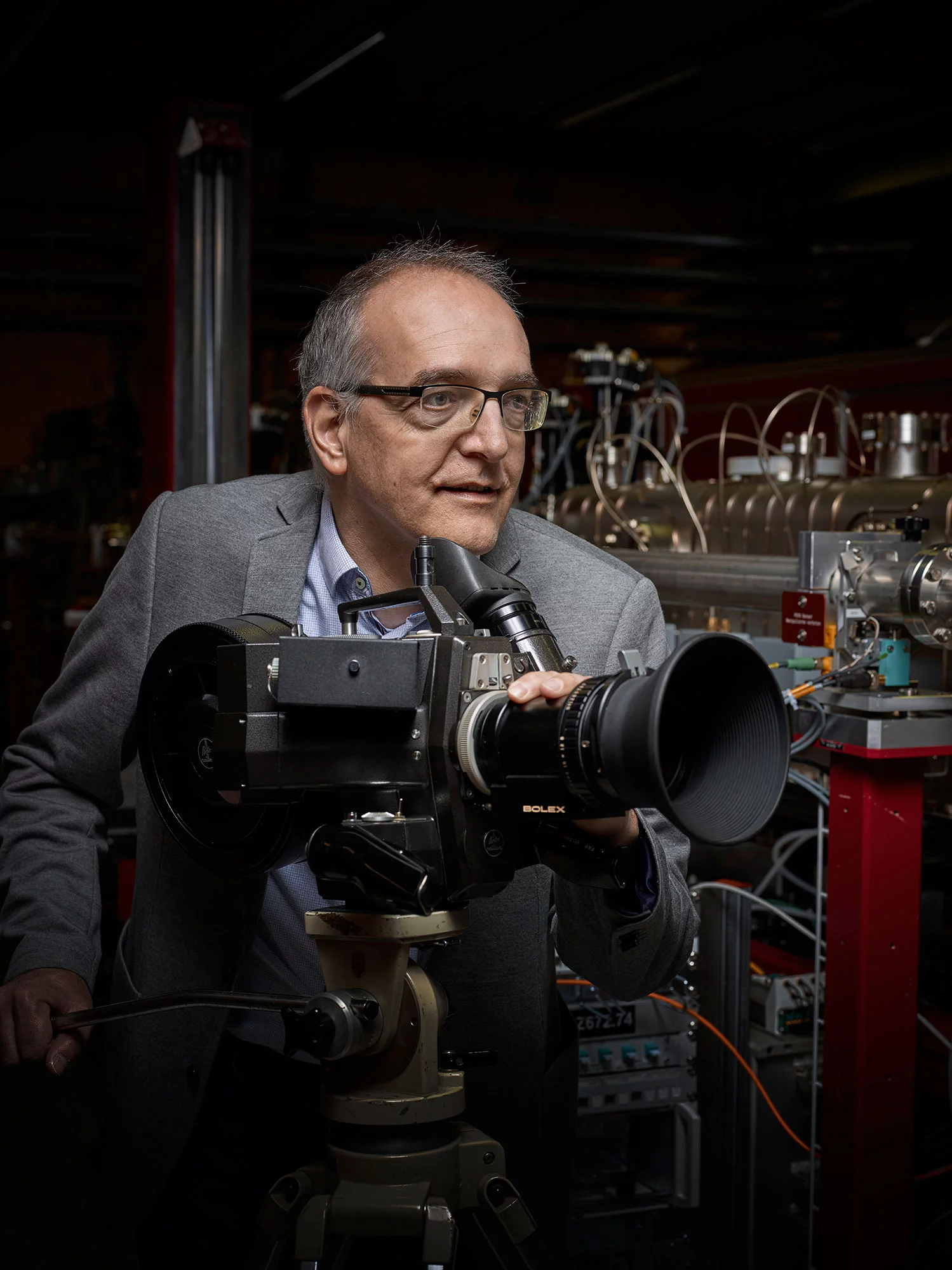
Luc Patthey stands next to a complicated arrangement of optical lenses, measuring instruments, countless cables, and a thin steel pipe. We're in Hollywood here. We're making movies
, explains the physicist with a smile: Our movie stars are molecules and materials.
As project leader, Luc Patthey was responsible for the optical components, the experiments, and the research program of the free-electron X-ray laser SwissFEL. He is enthusiastic about this unusual film studio: <q<With extremely short exposure time, we follow what is happening on the level of the atoms. This is made possible by the intense, ultrashort X-ray laser pulses that shoot through the steel pipe and flash light on the atomic processes.
With his team, Luc Patthey makes movies out of this fascinating microcosm. They are expected to contribute to producing better medicines, environmentally friendly energy technologies, or new materials for information technology. SwissFEL is a powerful machine
, says the project leader as he shows what lies behind the scenes: Before the X-ray pulses get to the two measurement stations, they run through several containers with mirrors that steer them exactly onto the right path.
Our movie stars are molecules and materials.
With an analogy, Luc Patthey illustrates how precisely their surfaces are polished in comparison to a normal mirror: If Switzerland was as flat as these mirror surfaces, the Alps between St.Gallen and Geneva would be just a few millimetres high.
A bold untertaking
Extreme precision pervades the entire SwissFEL building. In the 740-metre-long tunnel that runs through the forest of Würenlingen beneath a mound of earth, the X-ray laser pulses are created. Here electrons are accelerated to nearly the speed of light and sent on a slalom course until they emit the desired X-ray light – intense flashes with a duration of only 1 to 100 femtoseconds (thousandths of a trillionth of a second). Hans Braun is responsible for the accelerator. He recalls how this daring undertaking got its start: When we began the preliminary work for SwissFEL in 2008, it was still not clear if free-electron X-ray lasers would even work.
The world's first facility, at Stanford in California, didn't go into operation until 2009.
With SwissFEL the physicists were able to show that the necessary beam quality could also be achieved with a much smaller and less expensive accelerator than the one in California. They benefitted from the experience they had built up with the Swiss Light Source SLS at PSI. Ultrashort pulses could already be generated with SLS, although with significantly less intensity. Those were heroic experiments
, explains Rafael Abela, who oversaw the construction of SLS as well as SwissFEL: In those days we needed two weeks for measurements that we could accomplish later at the Stanford facility in ten minutes.
Watching how seeing begins
SwissFEL cost around 275 million Swiss francs – financed mainly by the federal government, with 30 million francs provided by the canton of Aargau out of its lottery fund. 275 million roughly corresponds to the production budget of the Disney film Pirates of the Caribbean – At World's End
with Johnny Depp in the lead role. In the planned SwissFEL movies, one of the stars is named rhodopsin. This protein can trap light and convert it into a chemical signal. Rhodopsin puts this capability to work in the human eye, where it serves as a light sensor and enables us to see even in dim light. Inside the rhodopsin molecule is a retinal, a form of vitamin A. In the dark, this elongate molecule has a flexed form; but when light falls on it, it stretches out and causes the whole rhodopsin to change its shape too. In humans, this change is a sign that light has arrived in the eye. Nerve pathways transmit the information to the brain, where the visual impression ultimately arises. The stretch reaction
of retinal is one of the fastest in biology and occurs within just 200 to 500 femtoseconds. With the X-ray laser, the researchers want to find out precisely how this stretching changes the rhodopsins and what role is played by the immediate surroundings of the retinal molecule inside rhodopsins.
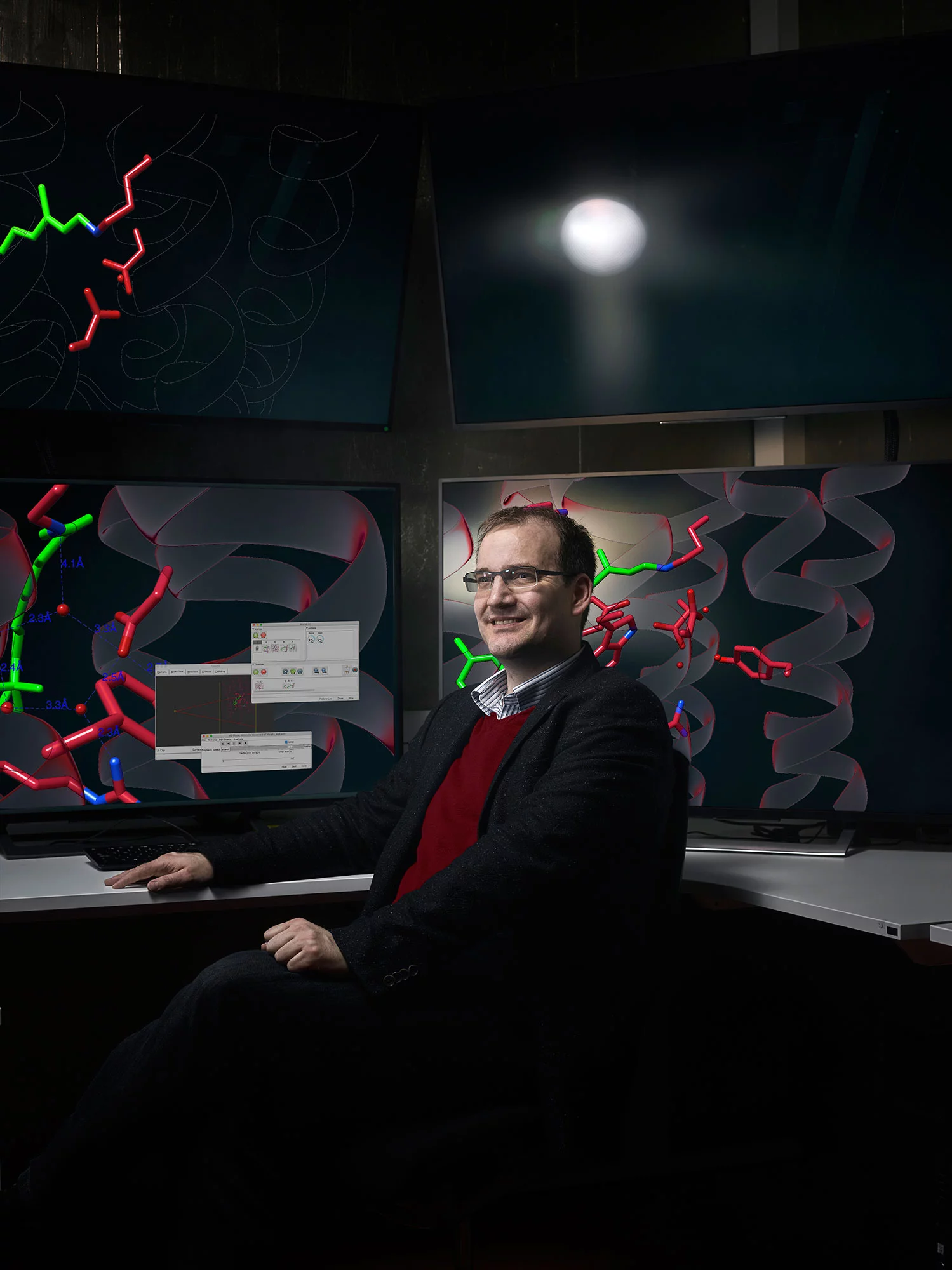
With SwissFEL we can watch the primary process of vision
, says Gebhard Schertler, head of the Biology and Chemistry Research Division at PSI and professor of structural biology at ETH Zurich: For someone like me, who has been working in the field for 40 years, this is extraordinarily fascinating.
That's why Gebhard Schertler relocated to Switzerland from Cambridge in England, where he worked at one of the world's most renowned research institutes. As research head he decides which experiments from his division are in the running for SwissFEL. When I've read the script, I know whether or not I want this movie.
Explaining his role, he says, In the theatre, maybe I would be the artistic director.
In the selection process, Schertler wants to concentrate in the future on films
in which the leading roles are played by molecules that are important for the effect of drugs.
Molecules in action
Jörg Standfuss, who likewise moved from Cambridge to PSI, has developed new technologies for the biology experiments and explains the process: The precursor materials are tiny purple crystals in which many exemplars of the rhodopsin protein are packed tightly in an ordered state.
The crystals are then sent individually into one of the experiment chambers of SwissFEL. There the X-ray flash hits the crystal and lights up the scene, which is recorded by the camera, and generates a single still image.
Because the intense X-ray pulse makes the crystal explode, new samples always have to follow. The experiment is set up in such a way that in the successive sample crystals, the retinal in the rhodopsin has advanced to a different stage in its extension. With that, the individual images show different phases of the motion and thus can be assembled into a movie. You need very many identical crystals
, says Jörg Standfuss: Normally proteins are frozen for measurements. But someone sitting in a block of ice can't move.
Therefore it is important for the experiment to take place at room temperature.
Jungfrau follows Pilatus
The high-speed X-ray camera is a detector that weighs around 70 kilograms. There is a tradition of constructing such instruments at PSI. One detector built for the particle physics laboratory CERN played a role in the confirmation of the Higgs boson. On that basis, the PSI physicists developed detectors for SLS, which today are manufactured by the company Dectris in Baden, Switzerland, and used at synchrotron facilities around the world. At the synchrotrons, the particles of the X-ray beams arrive at the detector in quick succession, yet one after the other
, explains Bernd Schmitt, head of the detector group at PSI: Therefore you can simply count these photons.
At SwissFEL, in contrast, the X-ray pulse is so intense and so short that this doesn't work any more. We need to measure the entire charge that the photons deposit in our detector and then calculate the number of photons.
The camera we developed for SwissFEL is named 'Jungfrau', and others are called 'Mythen' and 'Pilatus'. So the whole world knows where they come from.
Bernd Schmitt and his team christened the camera for SwissFEL Jungfrau
, after they already had developed Mythen
and Pilatus
for SLS. So the whole world knows where the detectors come from
, the physicist laughs. Leonardo Sala is responsible for recording the shots. The enormous quantities of data are a big challenge here
, he says, because several terabytes of data accumulate with every experiment: a flood of data full of hidden correlations, whose secrets are revealed only by means of complex methods.
From research to new medicine
The target audience for the SwissFEL movies also includes the pharmaceutical research community. Insights into the dynamics of biomolecules can help researchers explore new drugs. We are convinced of that
, says Gebhard Schertler. A conviction that must first find its way into implementation. That is why structural biologist Gebhard Schertler, physicist Rafael Abela, and former pharma research manager Michael Hennig founded a start-up co-located with PSI. Since 2015, the start-up leadXpro has been investigating so-called membrane proteins, a category that also includes the above-mentioned rhodopsins. Medicinal agents that fight illnesses such as cancer, infections, inflammation, or retinal diseases can dock on these proteins in a targeted way.
The better a drug molecule fits into the binding pocket of a membrane protein, the faster-working and more effective a medicine is, and the fewer side-effects it has. The modern approach in drug research presumes that one knows the structures of these membrane proteins and their bonds with potential new medicines. SwissFEL will open up new possibilities, because it also makes the motion of the membrane proteins visible
, says Rafael Abela, who spurred on the construction of SwissFEL. Already, leadXpro has been able to sign contracts with several large pharmaceutical companies and also to advance its own projects in cancer and antibiotics research. Two years after its founding, the firm has already developed very well, and it also profits from the talent pool of the science location Switzerland, the large-scale research facilities of PSI and electron microscopy at the University of Basel
, says leadXpro CEO Michael Henning.
Text: Barbara Vonarburg