Experiments at SwissFEL will help us understand important processes in living organisms. They will reveal how vital biomolecules, whose structures cannot be determined using current techniques – are constructed. They will also reveal how the shapes of these molecules change. This knowledge will help us understand disease processes and to develop the drugs needed to treat them.
Biological molecules perform a vast number of tasks inside living organisms – for example, they pass information arriving from a cell’s external environment to its interior, and help build the other vital substances it needs using its DNA as a blueprint. Each of these biomolecules works like a tiny high-precision machine, whose components need an exactly predetermined shape to be able to carry out their cellular functions.
A great deal of research effort aims at working out exactly how biomolecules are built up and how they "function". Research at SwissFEL will greatly broaden our understanding of biomolecules – by revealing molecular structures that cannot be determined using current methods, as well as showing these molecules "in motion" instead of merely in a resting state. Armed with this knowledge, it will be possible to understand basic processes in living organisms better, and to lay the foundations for new treatments for a range of diseases. It will also be possible to recognise how faulty molecules cause illnesses, and how novel medications will need to function in order to target key processes in pathogens like bacteria and viruses.
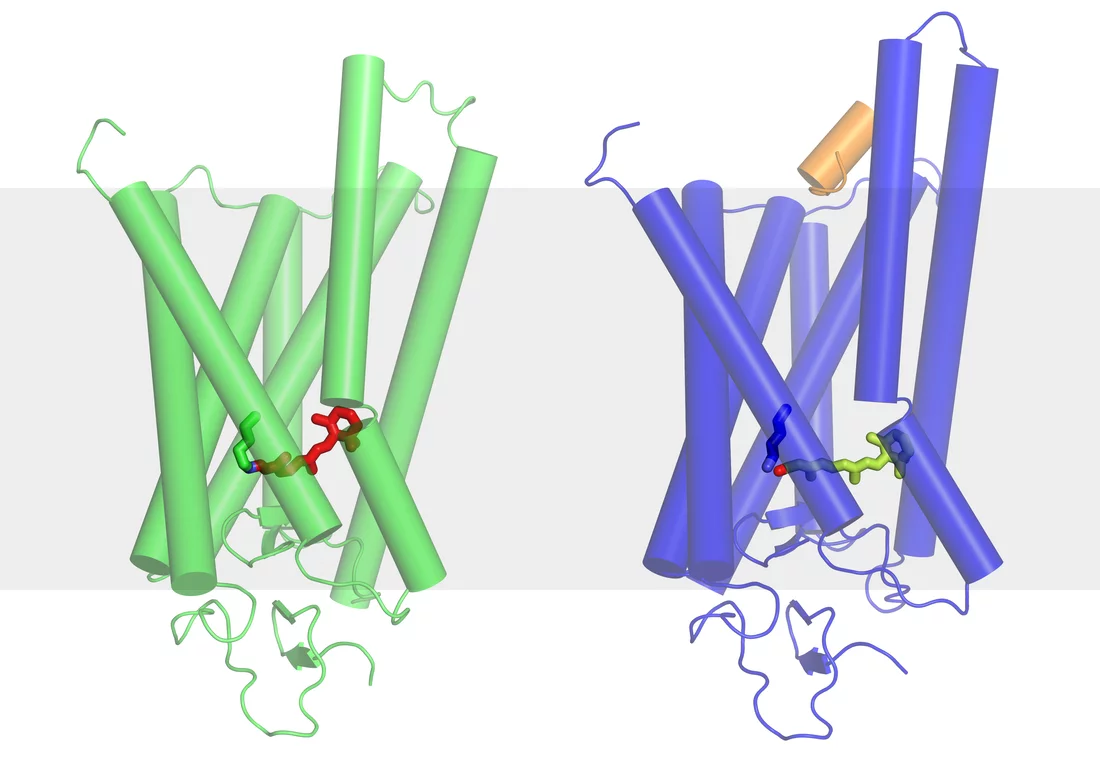
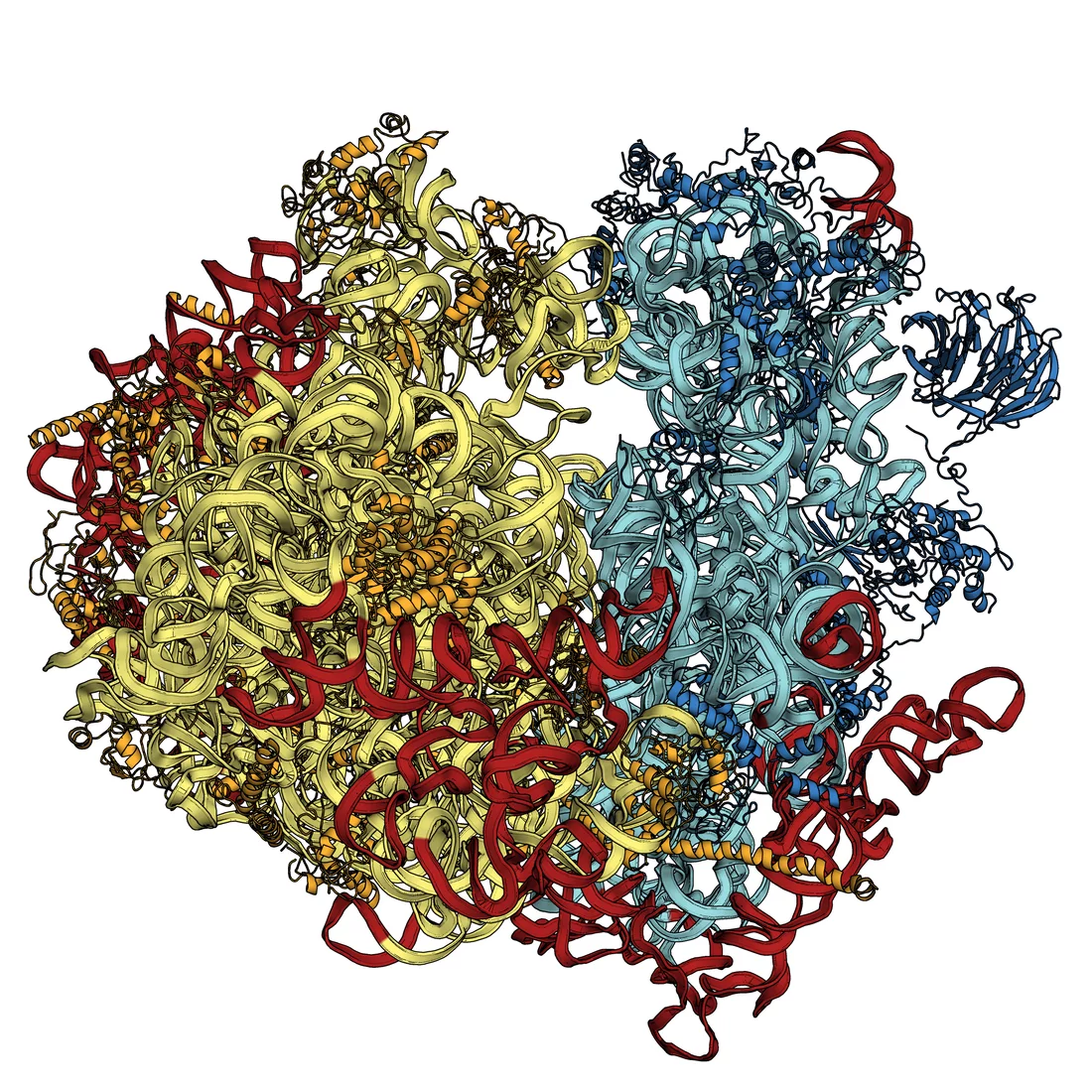
We can already determine the static structures of biomolecules by conducting experiments at the Swiss Light Source (SLS) at PSI, or at similar facilities – the manner in which ribosomes function was clarified in this way. Ribosomes in cells have the task of assembling proteins from individual amino acids, according to the pattern laid down in the DNA: this work was awarded the Nobel Prize for chemistry in 2009. The main drawback to the approach currently used at the SLS however, is that it requires use of protein crystals in which many thousands copies of the same molecule are arranged into a regular structure; for many molecules it is impossible to grow the crystals required. Those that have proved refractory to current approaches include many membrane proteins involved in controlling exchanges between the interior of a cell and its environment. Membrane proteins either fail to form crystals at all or only form crystals a few hundred nanometres in length, which is too small. At SwissFEL, it will be possible to determine the structure of these molecules.
The machinery of life in motion
Experiments at SwissFEL will make it possible to observe complex biomolecules in motion. When they are performing their various tasks, these molecules are undergoing continuous changes in shape. For example, light entering the eye stimulates the retinal protein rhodopsin to change its shape. This in turn triggers a chain of further chemical reactions that ultimately generate a nerve impulse, which is sent towards the brain. After a short time, the rhodopsin molecules return to their original shape and are ready to receive photons once more. Similar processes occur in most molecules. Even if the initial and final states of a molecule are known, there has not previously been a way of observing a molecule whilst it is actually undergoing a shape change. The extremely short X-ray light pulses of SwissFEL will now make it possible to see the intermediate states of a molecule, and thereby shed light on exactly how it functions.
Background information on research projects represented in these illustrations:
Understanding the nanomachines of life - Press Release on Ribosome Research (http://psi.ch/bwdu)Basic structures of sight deciphered (http://psi.ch/NT4t)
When the information bus in a cell breaks down - Press Release on Research on the Light Sensor Rhodopsin - in German only (http://psi.ch/nHkj)