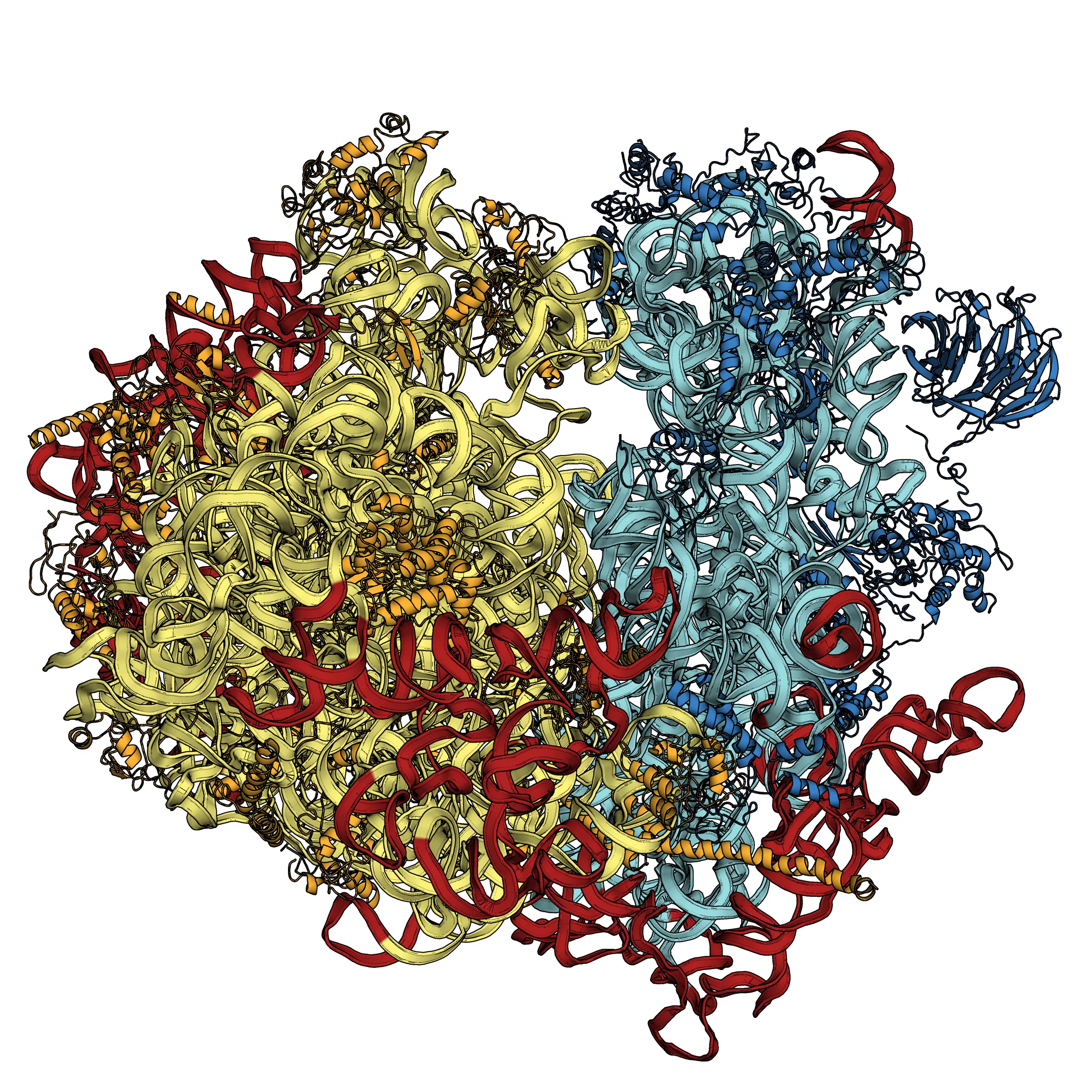
Structure of eukaryotic ribosomes resolved
Ribosomes are the protein factories of the living cell and themselves very complex biomolecules. Now, a French research group at the Institute of Genetics and Molecular and Cellular Biology, IGBMC, in Strasbourg (run jointly by CNRS, INSERM and Strasbourg University) has succeeded in determining the structure of the ribosome found in yeast cells. This is the first time that the ribosome structure has been determined in a eukaryotic cell – a complex cell containing a cell nucleus. Understanding ribosome structures not only contributes to our knowledge of biological processes, but can also help in the development of new medicines. The results have been published in the journal Science. An important part of the experiments was performed with synchrotron light at the Swiss Light Source SLS of the Paul Scherrer Institute.
In a living cell, ribosomes produce proteins – highly complex biomolecules responsible for countless tasks in the organism. The structures of proteins are set out in genetic information – DNA – and the ribosome’s task is to build the proteins according to this blueprint. Over the past few years, scientists managed to decipher the structure of the ribosomes of bacteria, and the Nobel Prize for Chemistry in 2009 was awarded for their achievement. Now, a group of scientists from the Institute of Genetics and Molecular and Cellular Biology in Strasbourg, France, has determined the structure of the ribosome in the more complex cell of yeast. In contrast to bacteria, yeast has eukaryotic cells, equipped with a cell nucleus. These ribosomes are also much larger, having a mass of 3.3 MDa instead of 2.3 MDa for the bacterial ribosome, where MDa stands for Mega-Dalton – a popular unit in molecular biology, corresponding to approximately one million times the mass of a hydrogen atom.
The French scientists determined the structure of the ribosome with a resolution of 0.415 nanometres (millionths of a millimetre). They also confirmed the existence of movements, not only within the ribosomal subunits but also between them, thus showing the importance of oscillations within the ribosome for protein synthesis. An important part of the investigations was performed at the Swiss Light Source, SLS, at the Paul Scherrer Institute. Here, the structures of complex biomolecules can be determined using synchrotron light. The SLS provides three high-performance beamlines for this kind of investigation, and one of the 2009 Nobel laureates is among SLS users. Additional services, such as the possibility of preparing the crystals necessary for the experiments, are also offered by this facility to academic and industrial researchers.
The next goal for the scientists is to determine the ribosome structure of further organisms, as well as to improve the resolution of these ribosomes and to describe their detailed three-dimensional structure – atom by atom. It can be expected that, for this, they will also decide to perform part of their experiments at the SLS. Detailed knowledge of the structure of the ribosome is not only important for our understanding of the fundamental processes of life, but also for the development of new pharmaceutical drugs. The working principle of some antibiotics is to block the protein synthesis in the ribosomes of bacteria. Thus, knowledge of the detailed structure of ribosomes in eukaryotic cells may help in the development of remedies against diseases caused by protozoa (such as malaria, toxoplasmosis, sleeping sickness, etc.), fungi, viruses and bacteria.
Based on the text of a press release published by CNRS.
About IGBMC
The Institute of Genetics and Molecular and Cellular Biology is a joint research unit of the French National Center for Scientific Research (CNRS), the French National Institute of Health and Medical Research (INSERM) and the University of Strasbourg. One of the leading European centres of biomedical research, it is devoted to the study of higher eukaryotic genomes and the control of genetic expression, as well as the analysis of the functioning of genes and proteins. This knowledge is applied to studies of human pathologies.
About PSI
The Paul Scherrer Institute develops, builds and operates large-scale, complex research facilities, and makes these facilities available to the national and international research community. The Institute’s own research focuses on solid-state physics and the materials sciences, elementary particle physics, biology and medicine, as well as research involving energy and the environment. With a workforce of 1400 and an annual budget of about 300 million CHF, PSI is the largest research institution in Switzerland.
Contact
Dr. Marat Yusupov, Institut de Genetique et de Biologie Moleculaire et Cellulaire, B.P. 10142, 67404 ILLKIRCH CEDEX, France,
Phone: +33 3 88 65 33 01, E-mail: marat@igbmc.u-strasbg.fr
Dr. Marcus Müller, Laboratory for Macromolecules and Bioimaging, Paul Scherrer Institute, 5232 Villigen PSI, Switzerland,
Phone: +41 56 310 5437, E-mail: marcus.mueller@psi.ch
Original publication
Crystal Structure of the Eukaryotic Ribosome
Adam Ben-Shem, Lasse Jenner, Gulnara Yusupova and Marat Yusupov,
Science 330, pp. 1203-1209 (26 November 2010)
DOI: 10.1126/science.1194294
Further information about protein-structure investigations at PSI
Studies on protein structures
Research using Synchrotron Light