Hydrogen fuel cells are an attractive technology for energy conversion, as they are considered to be a clean technology, particularly for motor cars. However, several technological challenges still need to be overcome if they are to gain a significant market presence. These include their service lifespan, which depends, amongst other things, on the robustness of the polymer membrane that acts as the electrolyte in a cell. Researchers at the Paul Scherrer Institute PSI have gained valuable insights into one of the most common mechanisms of degradation in these membranes.
An electrolyte is a material that conducts ions, that is, electrically charged atoms. In a hydrogen fuel cell, the electrolyte function is performed by a polymer membrane. This membrane also serves to separate hydrogen and oxygen, the two gases that are fed into the cell, so that no explosive gas is produced. The membrane of a hydrogen fuel cell conducts positively charged hydrogen ions (protons) from the anode to the cathode of the cell. The hydrogen fed into the cell is only ionized at the anode (negative electrode of the cell), these ions then migrate through the membrane electrolyte to the cathode (positively charged electrode), where they react with oxygen from air. From this reaction, electricity and – as a by-product – water, are produced.
The Water Dilemma
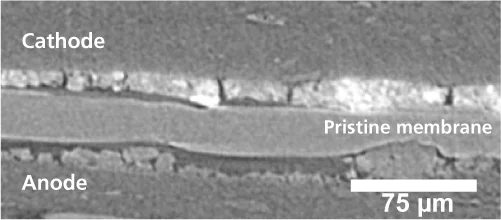
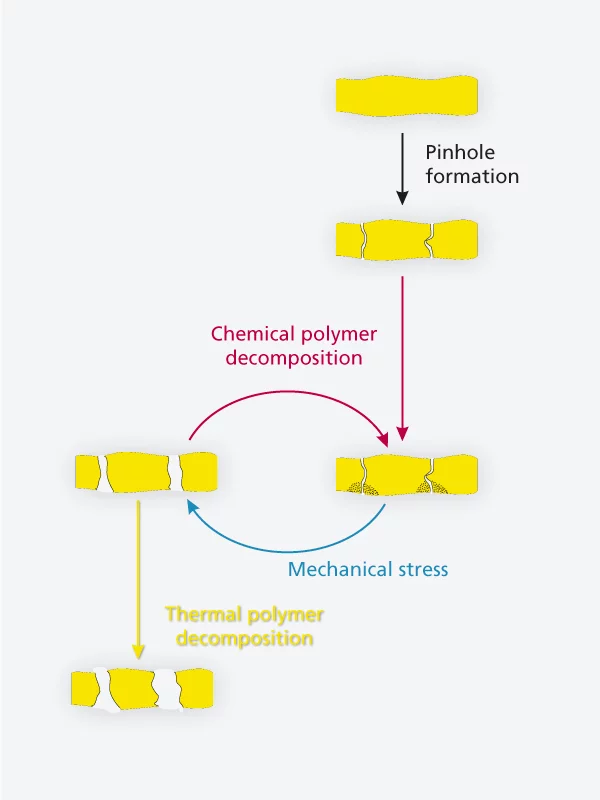
In the fuel cell, water is ubiquitous. It accounts for up to a quater of the weight of the membrane itself. The consequences of this are two-edged. Since water increases the conductivity of the polymers, it is seen as a welcome guest in the membrane. At the same time, moisture acts as a plasticiser, which sets membrane degradation in motion. PSI-researchers have now shown that at the beginning of the end of a hydrogen fuel cell, membranes frequently have specific faults, that are caused by operational changes in their water content. Researchers think of the degradation process in the following way: in the typical start- and stop- operations of a fuel cell, like those in a car driving through city traffic, a greater or lesser amount of power is produced over time. Thus the amount of the by-product of electricity generation - water – is variable. Variations in moisture (water content) lead to alternate shrinking and swelling, and therefore to deformation of the membrane. Researchers call this phenomenon ”creep“. In fact, after many operating cycles, the membrane can adopt, in places, a shape resembling a crawling caterpillar, as evidenced by X-ray images. At some points, this ”caterpillar“ becomes exposed to higher mechanical stress and is thus more susceptible to fracture. At the weak points, small ’pinholes‘ can now form. These holes are too small at first to inflict much damage as they typically occupy only a few parts per million of the membrane surface area, and their impact on fuel cell functioning at this stage is still too small to measure.
Pinholes are just the beginning of a vicious cycle
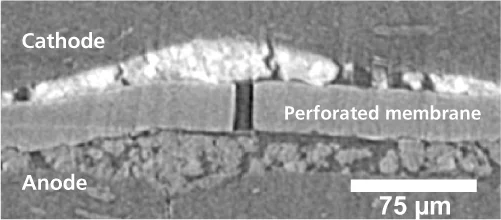
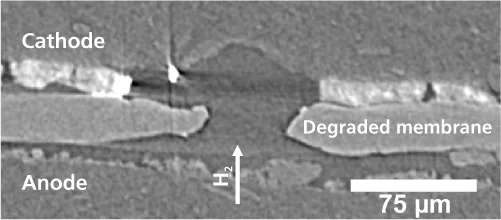
However, these small pinholes can start further problems, because gas molecules (hydrogen and oxygen) can pass through these open channels in the initially gas-tight membrane. Hence hydrogen molecules can now reach the cathode, where they can react with oxygen and form free radicals. These free radicals are highly reactive, and damage the membrane in the vicinity of the pinhole chemically. As a result the chains of carbon atoms from which polymer membranes are made get broken. And since shorter chains are less strong, the membrane fails far more easily under mechanical stress, and the holes grow larger. Then a vicious cycle sets in: more gas flows through these larger holes, and more free radicals are formed. Ultimately, the holes in the membrane are so large, that enough gas can pass through the membrane to ignite a combustion reaction between the oxygen and hydrogen. In a very short time, the heat released by the combustion can cause the membrane to melt and eventually the cell to fail. Experts refer to this as ”sudden death“. Until now, it has not be entirely clear whether combustion can occur inside a fuel cell after a gas leak caused by the combination of mechanical and chemical degradation. The present work by PSI-researchers has now demonstrated that combustion may well be ignited and thus represent the final stage of the fuel cell membrane degradation process.
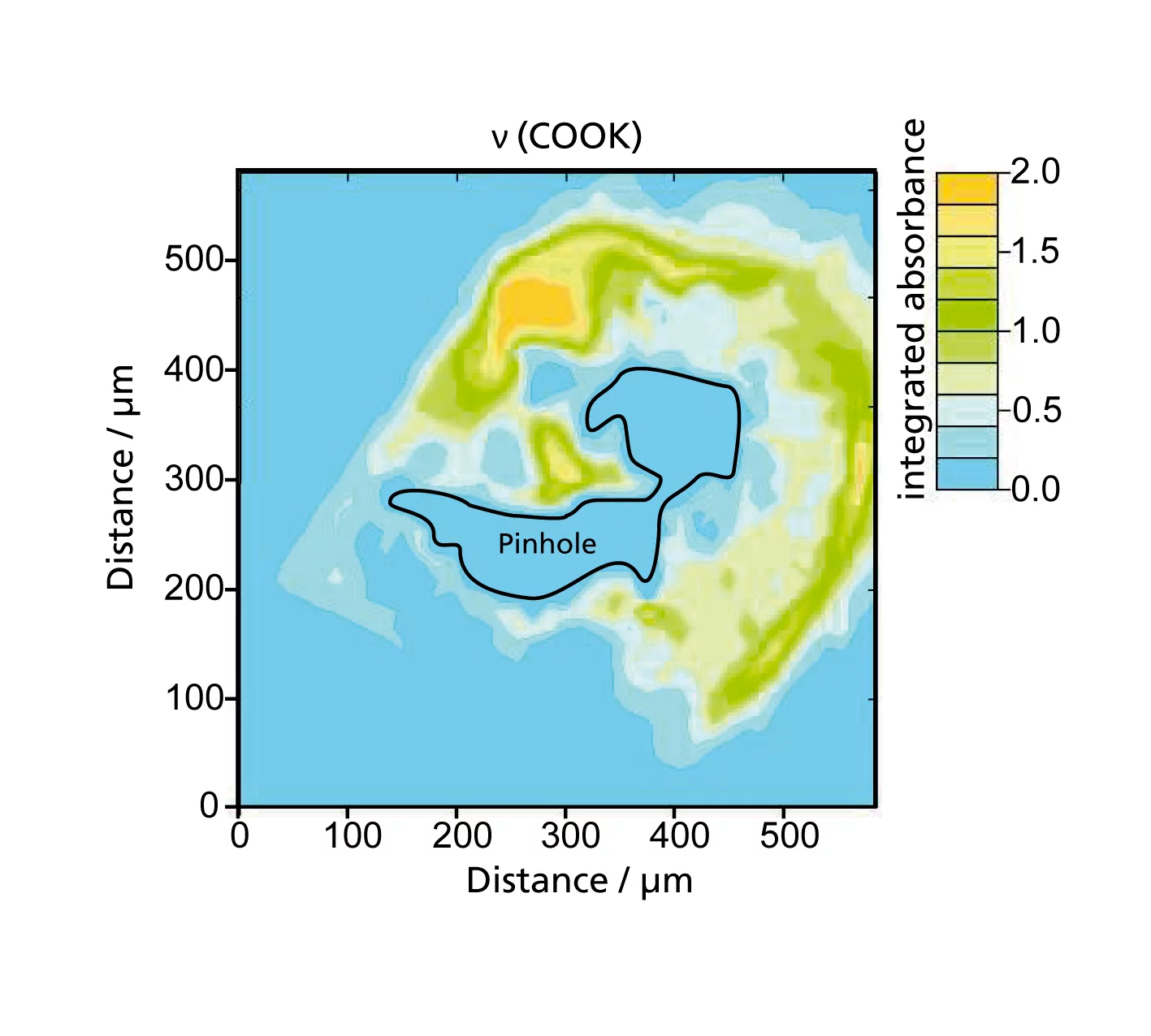
Elucidation thanks to X-ray and infrared light from the PSI-Synchrotron Light Source
The experiments that led to these findings formed the backbone of a doctoral dissertation by PSI-PhD student, Stefan Kreitmeier. Kreitmeier experimented with small sections of commercially available fuel cell membranes. Using ion beams, he drilled small micrometer-sized pinholes into the membranes. This operation was necessary, because under normal conditions, pinholes only appear in the membrane stochastic and slowly. The artificially created pinholes were all similar in shape and size and in the areas far from these holes the membrane was undamaged. This was confirmed by measurements using Infrared Spectromicroscopy and X-ray microtomography, which were carried out at the Swiss Light Source SLS. X-ray tomography offers the possibility of producing 3-D-images by taking lots of images from different angles – in this case, the sample was rotated – and combining them. Thanks to the high intensity of the X-rays from the SLS, high-resolution images could be created in a few seconds. Membranes prepared with pinholes were then subjected to a series of standard stress cycles, until the membranes failed. Then the researchers began their forensic work. They produced 2-D-maps of the chemical composition of damaged membrane areas. These chemical maps were also produced at the Infrared-Beamline at the SLS. This allowed the chemically damaged polymer molecules, which absorb infrared light particularly strongly, to be identified in the areas around the defects and provided crucial information about the degradation mechanism. The defects in the “dead“ membrane were then imaged in 3-D using X-ray microtomography at the SLS. The effects of combustion also came to light.
Implications for fuel cell operation
“These results could have practical implications for fuel cell operation“, said Kreitmeier’s Ph.D. Supervisor and Group Leader, Felix Büchi. The researchers have discovered that the gas permeability of the defects decreases with increased temperature or humidity. It is interesting to note that, depending on the size of the defect, water molecules were able to reseal the pinholes, which again made the defects gastight. Water has therefore been confirmed as having an ambiguous role, in both causing and solving the degradation puzzle. In the future, therefore, scientists may put more emphasis on answering the question of the right amount of water in a fuel cell.