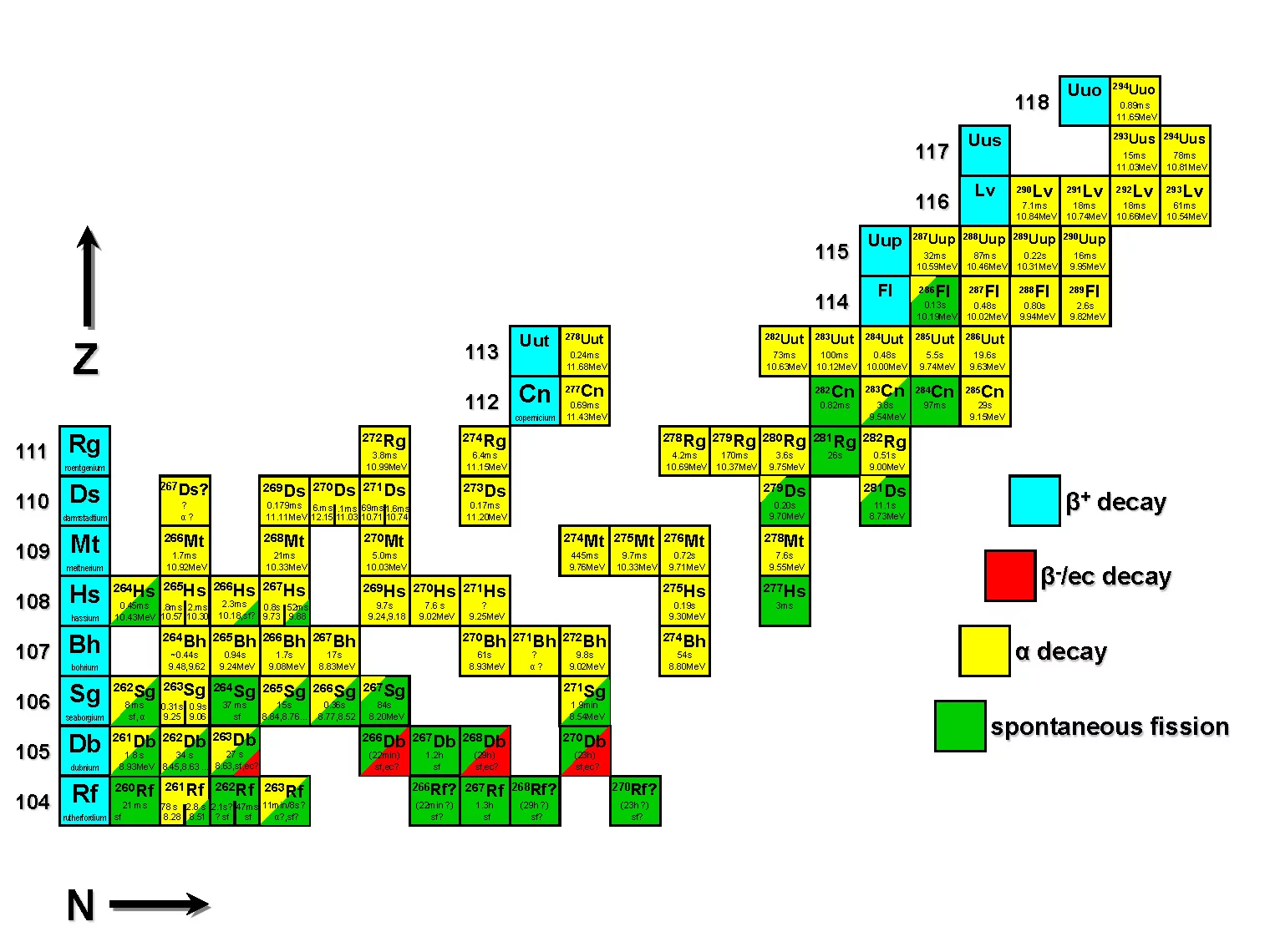
How a chemist investigates superheavy elements
Robert Eichler wants to determine the chemical properties of superheavy atoms. These are difficult to produce in the first place, however; and as soon as the scientists do manage to create one, it disintegrates again. Yet the research group leader and his colleagues refuse to give up – and test their measurement setup first with surrogate atoms. The dream: to create atoms one day that are still larger than all previously known and yet, despite that, do not decay rapidly. On the chemists' map of the elements, this would be a thus-far undiscovered island of stability.
Zis the number of protons in the atomic nucleus and, at the same time, the number of the element.
Nstands for the number of neutrons in the nucleus. All atoms discovered so far in this region are unstable and decay in one way or another (see colour coding). Gaps remain for the researchers to close – along with the possibility of one day discovering an
island of stabilitybeyond the elements listed here. (Image: Robert Eichler)
If the scientist Robert Eichler was an animal, then maybe a snake. Snakes need a meal only every few days. Eichler's requirements are similarly modest, when it comes to the objects of his research: This is partly because he has to wait several days to get one into his test chamber. Eichler and his colleagues study things as exotic as they are tiny – individual atoms of the superheavy elements.
Heavy
in this context is a relative term. It does not in the least refer to what we experience as heavy in everyday life, say, materials such as iron or lead. No, it has to do with the weight of the individual atoms a particular chemical element is made of (see info box: The periodic table of elements).
Artificial atoms
The superheavy atoms that Robert Eichler is stalking are obviously still too small for the human eye to see. And yet in comparison to their fellow atoms – those of oxygen, sulphur, or gold for example – they are so big and out of the ordinary that they do not occur in nature; they have to be produced under laboratory conditions with the help of large particle accelerators.
Dubna. This Russian city with 70,000 inhabitants lies a good hundred kilometres north of Moscow on the Volga River. The city's coat of arms shows a lot of water and an oak tree – dub
means oak
in Russian. A third motif emblazoned on the coat of arms is the symbol for an atom. That's because the Joint Institute for Nuclear Research is probably the city's most significant facility. Several particle accelerators are located here. One specialty of the institute in Dubna is producing atoms of superheavy elements and doing research with these weighty oddballs.
Childhood memories of Russia
Around 2500 external scientists come to the PSI every year to use large research facilities here that their home institutions lack. Robert Eichler and his colleagues from the PSI do it the other way around: They travel regularly to Dubna, together with their measurement setup, in order to carry out the part of their experimental work that is not possible at the PSI. The city's remote location doesn't bother Eichler. Quite the contrary, because he associates Dubna with childhood memories. Raised in the former GDR, Robert Eichler in his youth spent many years in this city on the Volga. His father, a scientist, was twice assigned for several years to the Joint Institute for Nuclear Research; the family accompanied him, and Eichler went to school in Dubna during this period. Through this background, I have a special relationship to the institute,
Eichler admits.
Many a young scientist appreciates the value of this international collaboration: It is not only that the superheavy elements are an exotic research area – in addition, collaboration with researchers who work within another cultural circle is attractive to younger colleagues and broadens their horizons,
says Eichler, who has headed the Heavy Elements Research Group at the PSI since 2002.
Here at the PSI, Eichler and his colleagues prepare everything in advance. They assemble their test chamber and install special electronics that have been developed at the PSI. In addition, Eichler's team develops and builds its own particle detectors, which withstand conditions hardly any other detector worldwide can handle. This part of the work makes our group multidisciplinary,
Eichler says. Engineers, technicians, physicists, and chemists work side by side here. It is helpful that all these people think differently. But it is also a bit of a challenge, because each discipline has its own set of scientific expressions. Sometimes we have to start out by finding a common language,
Eichler says with a smile.
When preparation of the test chamber is finished at the PSI, the researchers first test it with everyday atoms that are not quite as heavy. Eichler, a chemist, calls these surrogate atoms homologous
atoms. That means that their chemical behaviour should be very similar to that of the superheavy elements that he wants to experiment with later in Dubna. The noble gases – neon, argon, xenon – are one example of a group of homologous elements. They all make commercial signs glow, though in different colours. Elements that are homologous to each other stand together in the same column of the periodic table of elements. The homologous elements that Eichler uses are, for example, tungsten and thallium. Tungsten serves as a homologue for the superheavy seaborgium, thallium for the yet-to-be-named element 113 (see info box).
Chemistry with single atoms
But Eichler would be no true scientist if he were not also open to surprises: What we want to find out is if seaborgium really behaves as a homologue to tungsten and if element 113 really is a homologue to thallium.
If the superheavy elements behave differently from their putative homologues, explanations will have to be found. Then we would have to consider if, and if yes, why the architecture of the superheavy atoms deviates from the usual rules.
To find this out, Eichler and his team must carry out chemistry with the superheavy elements. And that in turn is unusual because chemical effects are normally considered en masse: What happens when this liquid is mixed with that one? That is the way classical chemical experiments run. Words like sextillion and septillion are needed to describe the numbers of atoms that interact with each other in these cases. It seems laughable that Eichler wants to carry out quite similar experiments with single atoms.
And yet it is not impossible. There absolutely is a lot we can do with these single atoms: Inside our test chamber we offer them a special surface and watch to see if, and if yes, how long they fasten themselves to it,
Eichler explains. That reveals something to us about the willingness of these atoms to form bonds, and that in turn is a central question of chemistry.
In further steps, Eichler and his colleagues can present the atoms with surfaces made from other materials, or vary the temperature of this surface.
Heavy atoms are unstable atoms
That Eichler and his colleagues – with considerable effort – are able to produce only single exemplars of their superheavy atoms is not even the whole problem. Another factor is that the atomic nuclei of this size are fundamentally unstable. Or to put it another way: These Atoms are radioactive and decay, shortly after they come into existence, into the smaller atoms of other elements. Thus Eichler and his colleagues are doing chemistry with radioactive atoms – in short: radiochemistry.
Asked if it isn't an unpleasant feeling to deal with this radioactivity, Eichler laughs: Not at all! We really are producing only single atoms. Naturally these decay radioactively. But in the concrete walls of our laboratory there's a mass of natural radioactivity many times higher than in this handful of atoms that we generate.
And there is yet another hurdle for Eichler's experiments: The larger an atomic nucleus is, the more unstable it tends to be – that means, all the more short-lived. The superheavy elements that Eichler studies have a median life span of half a second, others even less, only one-hundredth of a second. Thus when the experimenters have managed to create the rare object of their research, they have very little time to work with it.
However, Eichler does not see this as a disadvantage – on the contrary. Every radioactive element has its own, known median life span. In our experiments we can use that as an inner clock that automatically starts with the production of the atom and keeps on running,
Eichler says. Among other things, the average moment at which the atoms decay can confirm that the researchers have been studying the corresponding element.
Beyond the radioactive elements: The island of stability
The periodic table of elements is the chemists' map. Yet there is a second map, a second representation of the atomic species, which goes into greater depth. Technically speaking, for each chemical element there is not just one form its atoms can assume: Atoms can have somewhat more or somewhat less neutrons in their nucleus and still remain the same element. The map that represents this fact is the chart of nuclides, also known as isotopes.
The chart of nuclides is on the one hand essential for nuclear physicists, and on the other hand for all who study the superheavy elements. That's because there is a large blank space at this end of the chart of nuclides. And it is not yet clear whether it will stay blank forever because no more stable atomic configurations can be formed here, even in the laboratory, or whether there still may be – just offshore from the peninsula of the stable atoms – a lonely island of stability.
That is actually what scientists call it: the island of stability. Its existence has been suspected since the late 1960s. With an experienced gesture, Robert Eichler runs his finger over the large chart of nuclides that hangs on the wall in his office: Up here is where this island of stable atoms and isotopes could lie. We want to get there someday and see what we can find.
It is the pure spirit of discovery that drives Eichler onward in this question, together with many other physicists and chemists. Like seafarers in an earlier age, they want to explore the blank spaces on the map so that they can plot a more comprehensive and accurate picture of the world.
That's exactly how one of my colleagues has expressed it: We're doing world-charting research,
explains Eichler. And it's true: The general human striving for knowledge comes through in our research.
Text: Paul Scherrer Institute/Laura Hennemann
The periodic table of elements
The chemical elements from which all matter on our planet and in the rest of the universe is composed can be arranged according to increasing atomic size. Line by line and column by column, the so-called periodic table of elements unfolds – a chart of colourful rectangles that resides in the chemistry room of every school. Element number 8 is oxygen, number 16 is sulphur, 29 is copper, 47 silver, 79 gold. Alongside familiar names there are at least as many that only chemists are likely to encounter, such as beryllium (number 4), yttrium (39), or hafnium (72).These numbers can't readily get arbitrarily high, however. From number 84 on, the atomic nuclei are so large that they are fundamentally unstable. These atomic nuclei – and with them the atoms – decay. That means these elements are radioactive: They send out ionising radiation and disintegrate in the process into the smaller atoms of other elements. The familiar radioactive elements uranium (number 92) and plutonium (number 94) fall into this category.
Finally, the really big elements – number 95 and up – are so highly unstable that they can't be found at all in nature. With considerable effort, they can be artificially created in the laboratory – yet they immediately decay again into smaller atoms. The median life span of these elements typically amounts to less than one second.
The artificial creation of these elements has been possible only for a few decades and only with the help of large particle accelerators. And therefore it is only recently – in January 2016 – that the International Union of Pure and Applied Chemistry (IUPAC) officially expanded the periodic table of elements to include a few superheavy elements: Now numbers 113, 115, 117, and 118 are also listed. On June 8th 2016, the IUPAC has presented the following suggested names for these elements: Nihonium, Moscovium, Tennessine, and Oganesson.
Contact
Robert Eichler, Head of the research group Heavy Elements, Laboratory for Radiochemistry, Paul Scherrer InstituteTelephone: +41 56 310 41 20, e-mail: robert.eichler@psi.ch [German, English]