In accordance with Switzerland’s Atomic Energy Act, radioactive waste from medicine, industry, research and sometimes the operation and demolition of nuclear power plants is supposed to be disposed of in a deep geological repository for low and medium-level waste, where it is initially sealed away in cement materials for several thousand years. Researchers from the Paul Scherrer Institute PSI and the Karlsruhe Institute of Technology in Germany have now demonstrated how the cement restricts the freedom of movement for radioactive substances based on the example of uranium. The new findings improve our understanding of the processes that will take place in the deep repository.
According to Switzerland’s Atomic Energy Act, radioactive waste from nuclear power stations, medicine, industry and research has to be disposed of in deep geological repositories. Low and medium-level waste, such as from research, medicine or the operation and demolition of nuclear power stations, for instance, should be safely enclosed for at least 100,000 years. By the time this period has elapsed, the waste’s radioactivity will have dropped to the level of natural radiation. By contrast, the deep repository for highly active waste, such as used nuclear power station fuel elements, needs to seal it away safely for at least 1,000,000 years. Here, too, the radioactivity falls to the levels present in nature after this period.
Low and medium-level waste is already packed into cement-containing materials prior to its transportation to the deep repository. The cement is particularly important in the interim storage phase as it prevents the radioactivity from escaping during this period, which can last for decades. Moreover, all low and medium-level waste is processed for eventual deep storage. Part of this preparation involves cementing much of this waste in steel barrels. In accordance with Switzerland’s disposal concept, the waste packed in this way is transferred into concrete containers at the deep repository’s surface plant, where in turn the cavities between the barrels in the containers are filled with a cement material. The storage chambers in the deep repository where these containers are enclosed are also filled up with a special cement mortar after storage. However, all the cement encasements serve solely to transport or to simplify the storage of the radioactive waste. In the deep repository, the cement does not act as an actual barrier to prevent the radioactive substances from entering the environment. This barrier function is performed by the clay rock that makes up the deep repository. Nevertheless, the radioactive waste spends the first few thousand years in the environment dominated by cement before the latter dissolves in the groundwater. The clay rock then retains this groundwater, with all the radioactive substances, in the deep repository for the long term.
To calculate the details of the processes that take place in the deep repository, it is important to know how the radioactive substances behave in the cement and what state they are in when they eventually cross over into the clay rock from the cement.
Cement delays contact between radioactive substances and clay rock
Earlier measurements already indicated that cement heavily restricts the mobility of radioactive substances such as uranium. The reasons for this and the precise whereabouts of the substances in the cement, however, remained unclear. The new study now sheds light on the matter: uranium is held on the surface and inside the structure of cement minerals. The trapping in the inner structure of a particular cement mineral ensures that the cement retains the uranium for a very long time.
Trapped uranium
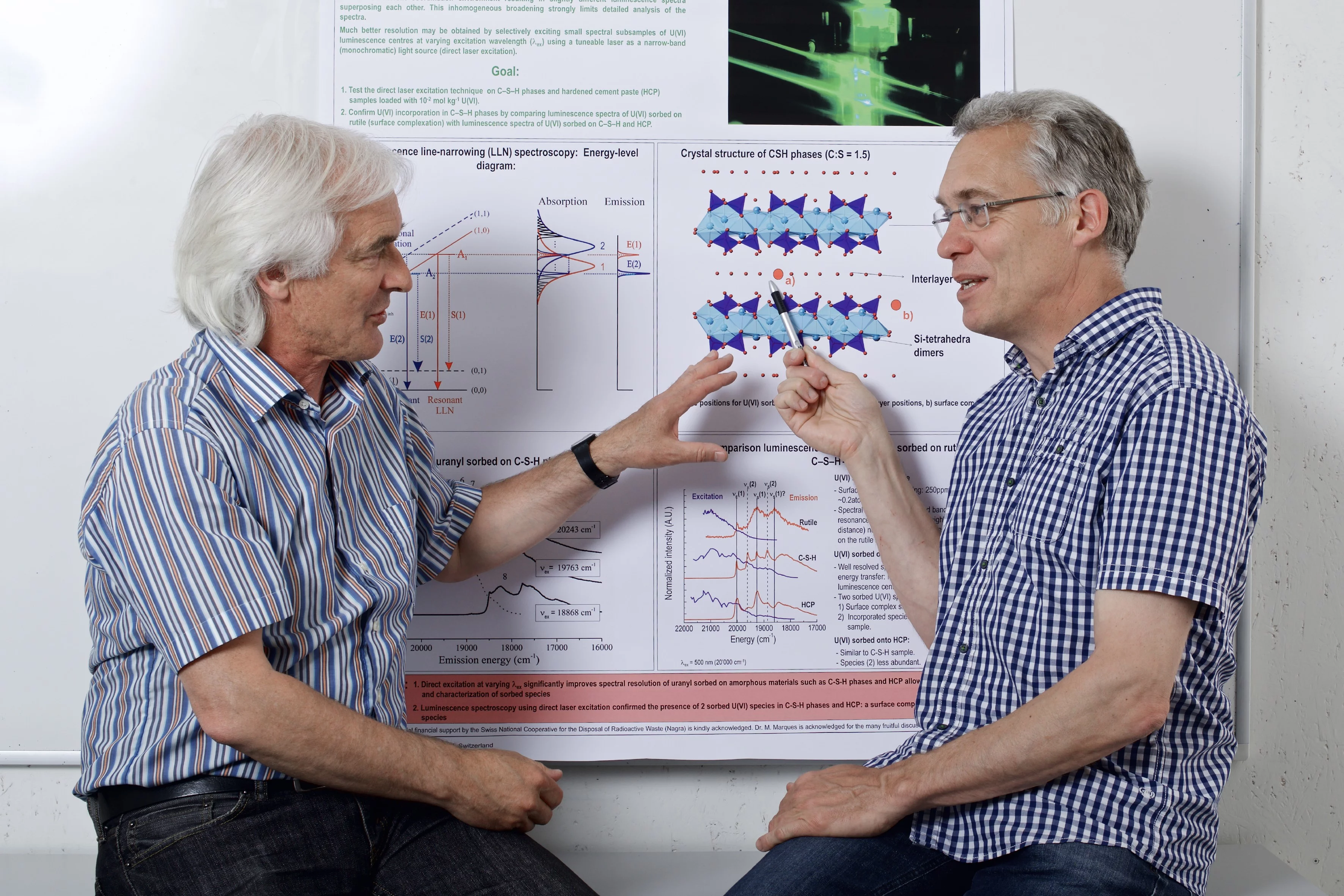
Cement is a mixture of several mineral types. One of these, which – as it now turns out – is especially crucial in a deep geological repository, is the class of so-called calcium silicate hydrates, which consist of the elements calcium, silicon, oxygen and hydrogen. Inside them, layers of calcium and silicon ions are separated by interlayers, where water molecules or calcium ions are usually found. As the new study reveals, uranium can enter between these interlayers and get bound there.
The scientists were able to demonstrate this with the aid of a laser-spectroscopic method, which involved directing laser light onto the uranium atoms. The latter initially absorb the laser light, which gives them an energy surplus; they are – to use the technical term – excited. The atoms shed the excess energy by emitting light themselves, the properties of which the researchers determine with a detector. From these measurements, the scientists are able to conclude where the uranium that absorbed, then emitted the light is located. The uranium that is bound on the surface of the cement minerals absorbs and emits light with a different energy (wavelength) to uranium trapped inside the structure of the cement minerals. The difference, however, is minimal, which is why it did not manifest itself clearly enough in previous experiments. The difference in energy was only rendered visible thanks to a measuring technique that PSI scientist Jan Tits developed to detect the incorporation of uranium. The measurements were conducted with a laser from the Nuclear Waste Institute at Karlsruhe Institute of Technology in Germany.
Better understanding of the role cement plays
The discovery that uranium is also enclosed in the interlayers in calcium silicate hydrates improves our understanding of the behaviour of radioactive substances in cement materials. It helps to describe the initial state for the long-term storage of radioactive waste in clay rock better. “Eventually, all cement minerals dissolve in the groundwater present in a deep geological repository. Of all these minerals, calcium silicate hydrates are actually the most stable, i.e. they resist the solvent effect of groundwater in the host rock in a deep geological repository for the longest time,” explains Erich Wieland, Head of the Cement Systems Group at PSI’s Laboratory for Waste Management.
Calcium silicate hydrates are found in cement in the form of tiny particles (nanoparticles). These particles are subject to constant change. The small particles break apart and form larger particles. During this growth process, uranium can become trapped in the structure of the calcium silicate hydrates, which means it is ultimately encased and thus shielded from the groundwater. The PSI researchers point out that this shielding is sufficient to delay the penetration of uranium and other radioactive substances into the clay rock in the deep repository for several thousand years.
Text: Paul Scherrer Institute/Leonid Leiva
Additional information
Laboratory for Waste Management at PSIContact
Dr Erich Wieland, Head of the Cement Systems Group, Laboratory for Waste Management, Paul Scherrer Institute, 5232 Villigen PSI, SwitzerlandTelephone: +41 56 310 2291, Email: erich.wieland@psi.ch
Dr Jan Tits, Cement Systems Group, Laboratory for Waste, Paul Scherrer Institute, 5232 Villigen PSI, Switzerland
Telephone: +41 56 310 4314, Email: jan.tits@psi.ch