Measurements at the Swiss Light Source SLS have helped to understand how the only known natural protein-mineral crystal is formed. It’s part of the fascinating glass-based skeleton of sponges. Those are some of the oldest animals on Earth and live in a wide range of waters, from lakes to deep oceans. The glass-based skeletons of some sponge species have intrigued researchers for a long time. How do the animals form a network of highly symmetrical glass scaffolds out of disordered glass? Researchers from TU Dresden together with the teams at the Paul Scherrer Institute PSI and the Center for Advancing Electronics Dresden are the first to determine the three-dimensional structure of a protein responsible for glass formation in sponges. The results are published in the journal Proceedings of the National Academy of Sciences of the USA.
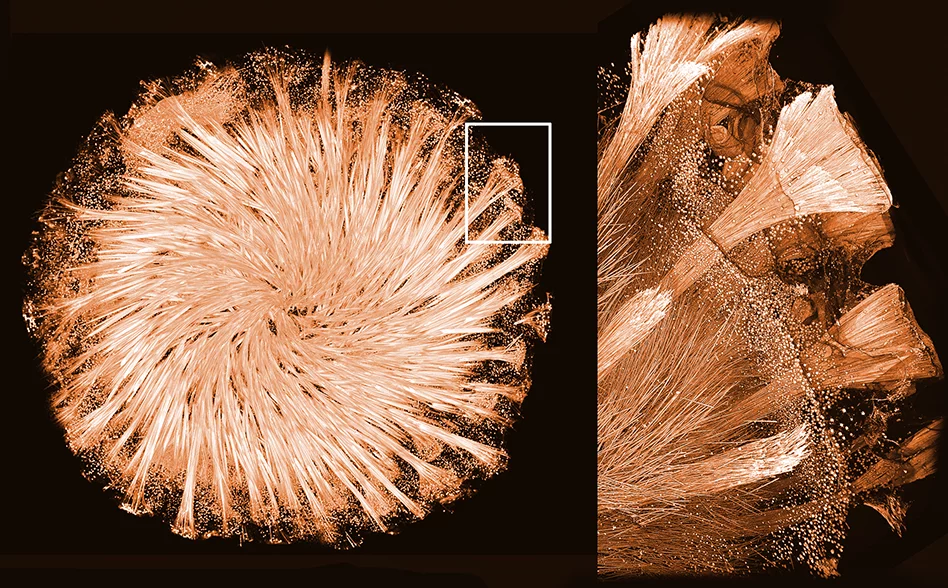
Glass sponges – as the name suggests – have a glass-based skeleton composed of a network of needles, hooks, stars, and spheres. To achieve such a unique architecture they have to manipulate the shape of disordered glass to form highly regular and symmetrical elements.
Thin crystalline fibres made of a protein, known as silicatein, are present in channels inside of these glass elements. It is known that silicatein crystals are responsible for glass synthesis in sponges and for shaping the glass skeleton. However, until now, efforts to determine the 3D structure of this protein to describe how it assembles into crystals and how those form the glass skeleton were unsuccessful. Mainly, because nobody was able to grow these crystals in the lab.
A team of researchers led by Igor Zlotnikov from the B CUBE – Center for Molecular Bioengineering at TU Dresden tried a different approach. Instead of producing silicatein in the lab and trying to obtain lab-grown crystals to study the structure, the researchers took the glass needles from a sponge skeleton and analysed the tiny crystals that already exist inside. However, as the crystals are so tiny, a few tricks were necessary to obtain their structure. Here, the team at PSI’s Swiss Light Source SLS were able to help.
Many tiny crystals instead of a big one
A traditional way of deciphering a 3D structure of a protein is to expose its crystal to a beam of X-rays. Atoms inside a protein crystal scatter the X-ray light providing a unique snapshot of its internal arrangement. By rotating the crystal and collecting such snapshots from many angles, the researchers can get enough data to decipher the 3D protein structure using computational methods.
Such an approach is widely used for crystals of at least 10 microns in size. However, the Zlotnikov group wanted to analyse silicatein crystals that were about 10 times smaller. When exposed to X-rays they were almost immediately damaged, making it impossible to collect a complete data set of snapshots from multiple angles.
With support from the team at SLS, the researchers used a new emerging method known as serial crystallography. “You combine diffraction images from many crystals,” says Filip Leonarski, beamline scientists at PSI, who was involved in the study. “With the traditional method you shoot a movie; with the new method you get many snapshot which you combine afterwards to decipher the structure.” Each snapshot is taken at a different part of the tiny crystal or even from a different crystal.
In total, the researchers collected more than 3500 individual X-ray diffraction snapshots from 90 glass needles at completely random orientations. Using state-of-the-art computational methods, they were able to find order within the chaos and assemble the data to determine the first complete 3D structure of silicatein. “The data analysis took some effort,” Leonarski says. “Luckily, we have enough experience with this method because we use it a lot at the X-Ray Free-Electron Laser SwissFEL.”
Protein-glass superstructure
Using the newly obtained 3D structure of silicatein, the researchers were able to understand its assembly and function inside the glass skeleton of the sponge. Additionally, the Zlotnikov group together with researchers from the Center for Advancing Electronics Dresden (cfaed) used high-resolution transmission electron microscopy (HRTEM) to take a closer look at silicatein crystals packed inside the glass needles.
“We have observed an exceptionally ordered and at the same time complex structure,” explains Zlotnikov. “Analysing the sample we have seen that it is a mixture of an organic and inorganic matter. Meaning that both proteins and glass form a hybrid superstructure that somehow shapes the skeleton of sponges.” He names the structure a functional 3D protein-glass superstructure, which was the first to be ever described in a living organism.
Based on a media release by TU Dresden with additions from the Paul Scherrer Institute/Brigitte Osterath
Link: https://tu-dresden.de/tu-dresden/newsportal/news/ordnung-im-chaos-finde…
Contact
Dr. Filip Leonarski
Laboratory for Macromolecules and Bioimaging
Paul Scherrer Institute, Forschungsstrasse 111, 5232 Villigen PSI, Switzerland
Telephone: +41 56 310 56 52, e-mail: filip.leonarski@psi.ch [English, Polish]
Dr. Igor Zlotnikov
B CUBE - Center for Molecular Bioengineering
Technical University Dresden, Tatzberg 41, 01307 Dresden, Germany
Telephone: +49 351 463 43090, e-mail: igor.zlotnikov@tu-dresden.de
Original publication
Natural hybrid silica/protein superstructure at atomic resolution
S. Görlich, A.J. Samuel, R.J. Best, R. Seidel, J. Vacelet, F.K. Leonarski, T. Tomizaki, B. Rellinghaus, D. Pohl, I. Zlotnikov
Proceedings of the National Academy of Sciences of the USA, 23 November 2020 (online)
DOI: 10.1073/pnas.2019140117
Copyright
PSI provides image and/or video material free of charge for media coverage of the content of the above text. Use of this material for other purposes is not permitted. This also includes the transfer of the image and video material into databases as well as sale by third parties.